What is Global Veterinary Drug Raw Materials Market?
The Global Veterinary Drug Raw Materials Market refers to the worldwide industry involved in the production and distribution of raw materials used to manufacture veterinary drugs. These raw materials are essential components in the creation of medications that treat various ailments in animals, including livestock and pets. The market encompasses a wide range of substances, such as active pharmaceutical ingredients (APIs), excipients, and other chemical compounds that are crucial for the formulation of veterinary drugs. The demand for these raw materials is driven by the increasing need for effective animal healthcare solutions, advancements in veterinary medicine, and the growing awareness of animal health and welfare. The market is characterized by a diverse range of suppliers and manufacturers who provide high-quality raw materials to pharmaceutical companies, ensuring the production of safe and effective veterinary drugs. As the global population of livestock and pets continues to grow, the demand for veterinary drug raw materials is expected to rise, making this market a vital component of the broader animal healthcare industry.
Antimicrobials, Antiparasitic Drugs, Antipyretic, Analgesic and Anti-inflammatory Drugs, Other in the Global Veterinary Drug Raw Materials Market:
Antimicrobials, antiparasitic drugs, antipyretic, analgesic, and anti-inflammatory drugs are key categories within the Global Veterinary Drug Raw Materials Market. Antimicrobials are substances used to kill or inhibit the growth of microorganisms, such as bacteria, fungi, and viruses, which can cause infections in animals. These raw materials are crucial for the production of antibiotics and other antimicrobial agents that help in treating bacterial infections in livestock and pets. Antiparasitic drugs, on the other hand, are used to eliminate or control parasites that infest animals, such as worms, ticks, and fleas. These raw materials are essential for the formulation of dewormers, insecticides, and other antiparasitic medications that protect animals from parasitic diseases. Antipyretic drugs are used to reduce fever in animals, while analgesic drugs are used to relieve pain. These raw materials are vital for the production of medications that help manage pain and fever in animals, ensuring their comfort and well-being. Anti-inflammatory drugs are used to reduce inflammation and swelling in animals, which can be caused by various conditions such as arthritis, injuries, and infections. These raw materials are important for the formulation of medications that help manage inflammation and improve the overall health of animals. Other raw materials in the veterinary drug market include vitamins, minerals, and other nutritional supplements that support the overall health and well-being of animals. These raw materials are used to produce a wide range of veterinary drugs and supplements that help maintain the health and vitality of livestock and pets. The demand for these raw materials is driven by the increasing need for effective animal healthcare solutions, advancements in veterinary medicine, and the growing awareness of animal health and welfare. As the global population of livestock and pets continues to grow, the demand for veterinary drug raw materials is expected to rise, making this market a vital component of the broader animal healthcare industry.
Livestock Industry, Pet Industry in the Global Veterinary Drug Raw Materials Market:
The usage of Global Veterinary Drug Raw Materials Market in the livestock industry is crucial for maintaining the health and productivity of farm animals. Livestock, such as cattle, pigs, sheep, and poultry, are susceptible to various diseases and infections that can impact their growth, reproduction, and overall productivity. Veterinary drugs formulated from high-quality raw materials are essential for preventing and treating these diseases, ensuring the well-being of livestock. Antimicrobials, for instance, are widely used in the livestock industry to treat bacterial infections and prevent the spread of diseases within herds and flocks. Antiparasitic drugs are also commonly used to control internal and external parasites that can affect the health and productivity of livestock. These drugs help in maintaining the overall health of farm animals, leading to improved meat, milk, and egg production. Additionally, antipyretic, analgesic, and anti-inflammatory drugs are used to manage pain, fever, and inflammation in livestock, ensuring their comfort and well-being. The use of these veterinary drugs is essential for maintaining the health and productivity of farm animals, which in turn supports the profitability and sustainability of the livestock industry. In the pet industry, the usage of veterinary drug raw materials is equally important for ensuring the health and well-being of companion animals. Pets, such as dogs, cats, birds, and small mammals, are susceptible to various diseases and health conditions that require effective treatment and management. Veterinary drugs formulated from high-quality raw materials are essential for treating infections, controlling parasites, managing pain and inflammation, and supporting the overall health of pets. Antimicrobials are commonly used in the pet industry to treat bacterial infections, while antiparasitic drugs are used to control fleas, ticks, and other parasites that can affect the health of pets. Antipyretic, analgesic, and anti-inflammatory drugs are also used to manage pain, fever, and inflammation in pets, ensuring their comfort and well-being. Additionally, vitamins, minerals, and other nutritional supplements are used to support the overall health and vitality of pets, helping them lead healthy and active lives. The demand for veterinary drug raw materials in the pet industry is driven by the increasing pet ownership, growing awareness of pet health and welfare, and advancements in veterinary medicine. As more people consider pets as part of their families, the demand for high-quality veterinary drugs and supplements is expected to rise, making the Global Veterinary Drug Raw Materials Market a vital component of the pet industry.
Global Veterinary Drug Raw Materials Market Outlook:
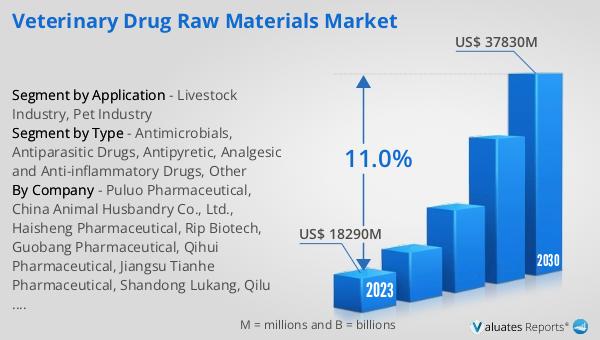
The global Veterinary Drug Raw Materials market was valued at US$ 18,290 million in 2023 and is anticipated to reach US$ 37,830 million by 2030, witnessing a CAGR of 11.0% during the forecast period 2024-2030. This significant growth reflects the increasing demand for veterinary drugs and the essential raw materials required for their production. The market's expansion is driven by factors such as the rising global population of livestock and pets, advancements in veterinary medicine, and the growing awareness of animal health and welfare. As the need for effective animal healthcare solutions continues to grow, the demand for high-quality raw materials used in the formulation of veterinary drugs is expected to rise. This market outlook highlights the importance of the Veterinary Drug Raw Materials market in supporting the production of safe and effective veterinary drugs, ensuring the health and well-being of animals worldwide.
| Report Metric | Details |
| Report Name | Veterinary Drug Raw Materials Market |
| Accounted market size in 2023 | US$ 18290 million |
| Forecasted market size in 2030 | US$ 37830 million |
| CAGR | 11.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Puluo Pharmaceutical, China Animal Husbandry Co., Ltd., Haisheng Pharmaceutical, Rip Biotech, Guobang Pharmaceutical, Qihui Pharmaceutical, Jiangsu Tianhe Pharmaceutical, Shandong Lukang, Qilu Pharmaceutical, LianBern Pharmaceuticals, Hanjiang Pharmaceutical, victory creature, Jiuzhou Pharmaceutical, Hui Sheng Biology, Xinhua Pharmaceutical, SeQuent Scientific Limited, Menadiona, S. L, Lasa Supergenerics Limited, Shreeji Pharma Internationa, Excel Industries Ltd., SUANFARMA, FIS Fabbrica Italiana Sintetici SpA |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |