What is Medical Device Inspection Services - Global Market?
Medical Device Inspection Services are a crucial component of the healthcare industry, ensuring that medical devices meet stringent safety and quality standards before they reach the market. These services involve a comprehensive evaluation of medical devices, ranging from simple tools like thermometers to complex machinery such as MRI machines. The inspection process includes a thorough examination of the device's design, functionality, and compliance with regulatory standards. This is vital because medical devices play a critical role in patient care, and any malfunction or defect can have serious consequences. The global market for these services is expanding as healthcare providers and manufacturers strive to maintain high standards of safety and efficacy. With advancements in technology and increasing regulatory requirements, the demand for medical device inspection services is expected to grow, ensuring that devices are safe for use and meet the necessary quality benchmarks. This growth is driven by the need to minimize risks associated with medical devices and to ensure that they perform as intended, ultimately safeguarding patient health and well-being.
Active Medical Devices, Non-Active Medical Devices in the Medical Device Inspection Services - Global Market:
Active medical devices are those that require a source of electrical energy or any power source other than that directly generated by the human body or gravity to function. These devices include pacemakers, defibrillators, and infusion pumps, among others. The inspection of active medical devices is particularly critical due to their complex nature and the potential risks associated with their malfunction. Inspection services for these devices involve rigorous testing of their electrical components, software, and overall functionality to ensure they operate safely and effectively. This includes assessing their ability to withstand various environmental conditions and their compatibility with other medical equipment. On the other hand, non-active medical devices do not rely on any external power source to function. These include surgical instruments, bandages, and orthopedic implants. The inspection of non-active devices focuses on their physical properties, such as material composition, durability, and sterility. Ensuring the quality of these devices is crucial as they come into direct contact with patients and play a significant role in treatment and recovery. The global market for medical device inspection services encompasses both active and non-active devices, with each category requiring specialized expertise and methodologies to ensure compliance with international standards and regulations. As the healthcare industry continues to evolve, the inspection services market is poised to grow, driven by the increasing complexity of medical devices and the need for stringent quality control measures. This growth is further fueled by the rising demand for innovative medical technologies and the expansion of healthcare infrastructure worldwide. The inspection services market plays a pivotal role in facilitating the safe and effective use of medical devices, ultimately contributing to improved patient outcomes and enhanced healthcare delivery.
Hospital, Ambulatory Surgery Center in the Medical Device Inspection Services - Global Market:
Medical device inspection services are integral to the functioning of hospitals and ambulatory surgery centers, ensuring that the devices used in these settings are safe, reliable, and effective. In hospitals, a wide range of medical devices are utilized daily, from diagnostic equipment like X-ray machines and CT scanners to therapeutic devices such as ventilators and dialysis machines. The inspection of these devices is crucial to maintaining their performance and preventing any potential malfunctions that could jeopardize patient safety. Regular inspections help identify any wear and tear, calibration issues, or software malfunctions, allowing for timely maintenance and repairs. This not only ensures the safety of patients but also enhances the efficiency of hospital operations by minimizing downtime and preventing costly equipment failures. Similarly, in ambulatory surgery centers, where outpatient surgical procedures are performed, the reliability of medical devices is paramount. These centers rely on a variety of devices, including surgical instruments, anesthesia machines, and monitoring equipment, to provide high-quality care to patients. Inspection services in these settings focus on ensuring that all devices are in optimal working condition, reducing the risk of complications during surgical procedures. By adhering to strict inspection protocols, ambulatory surgery centers can maintain high standards of patient care and safety, ultimately improving patient outcomes and satisfaction. The global market for medical device inspection services is thus essential in supporting the healthcare industry, providing the necessary expertise and resources to ensure that medical devices are safe, effective, and compliant with regulatory standards. As healthcare facilities continue to adopt advanced medical technologies, the demand for inspection services is expected to grow, underscoring their importance in the delivery of quality healthcare.
Medical Device Inspection Services - Global Market Outlook:
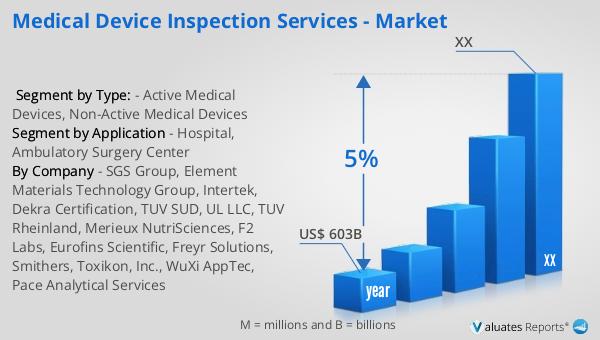
Based on our analysis, the worldwide market for medical devices is projected to reach approximately $603 billion in 2023, with an anticipated growth rate of 5% annually over the next six years. This growth trajectory highlights the increasing demand for medical devices across various healthcare sectors, driven by technological advancements and the rising prevalence of chronic diseases. The expansion of the medical device market is also fueled by the growing aging population, which requires more healthcare services and medical interventions. As a result, there is a heightened need for innovative and efficient medical devices that can improve patient care and outcomes. The projected growth rate of 5% reflects the dynamic nature of the medical device industry, characterized by continuous innovation and the introduction of new products that cater to the evolving needs of healthcare providers and patients. This growth is further supported by the increasing investments in healthcare infrastructure and the rising focus on personalized medicine, which requires specialized medical devices for diagnosis and treatment. As the global market for medical devices continues to expand, the role of medical device inspection services becomes even more critical, ensuring that these devices meet the highest standards of safety and quality. By providing comprehensive inspection and testing services, the industry can support the development and deployment of cutting-edge medical technologies that enhance patient care and improve healthcare delivery worldwide.
| Report Metric | Details |
| Report Name | Medical Device Inspection Services - Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | SGS Group, Element Materials Technology Group, Intertek, Dekra Certification, TUV SUD, UL LLC, TUV Rheinland, Merieux NutriSciences, F2 Labs, Eurofins Scientific, Freyr Solutions, Smithers, Toxikon, Inc., WuXi AppTec, Pace Analytical Services |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
