What is Global Adalimumab Biosimilars Market?
The Global Adalimumab Biosimilars Market refers to the worldwide market for biosimilar versions of Adalimumab, a biologic medication primarily used to treat various autoimmune diseases. Biosimilars are essentially highly similar versions of original biologic drugs, developed after the original product's patent expires. Adalimumab, originally marketed under the brand name Humira, is used to treat conditions such as rheumatoid arthritis, Crohn's disease, ulcerative colitis, and psoriasis. The market for Adalimumab biosimilars has grown significantly due to the high cost of the original biologic drug and the increasing prevalence of autoimmune diseases. These biosimilars offer a more affordable alternative while maintaining similar efficacy and safety profiles. The market encompasses various regions, including North America, Europe, Asia-Pacific, and the rest of the world, with key players continuously investing in research and development to improve these biosimilar products. The growth of this market is driven by factors such as the rising incidence of chronic diseases, the need for cost-effective treatment options, and favorable regulatory policies that encourage the development and approval of biosimilars.
80mg, 40mg, 20mg in the Global Adalimumab Biosimilars Market:
In the Global Adalimumab Biosimilars Market, the dosage forms of 80mg, 40mg, and 20mg play a crucial role in the treatment of various autoimmune diseases. The 80mg dosage is typically used for patients who require a higher dose due to the severity of their condition or for those who have developed a tolerance to lower doses. This higher dosage form is often administered less frequently, which can be more convenient for patients who prefer fewer injections. The 40mg dosage is the most commonly prescribed form and is used for a wide range of conditions, including rheumatoid arthritis, Crohn's disease, and psoriasis. This dosage is usually administered every two weeks and is effective in managing symptoms and preventing disease progression. The 20mg dosage is generally used for pediatric patients or adults who require a lower dose due to factors such as body weight or the presence of other medical conditions. This lower dosage form is also beneficial for patients who may experience side effects at higher doses. The availability of these different dosage forms allows healthcare providers to tailor treatment plans to the specific needs of each patient, ensuring optimal efficacy and safety. The development and approval of these biosimilar dosages are subject to rigorous regulatory standards to ensure they meet the same quality, safety, and efficacy requirements as the original biologic drug. The manufacturing process for these biosimilars involves advanced biotechnological methods to produce highly similar versions of the original Adalimumab, ensuring that patients receive the same therapeutic benefits. The market for these dosage forms is highly competitive, with numerous pharmaceutical companies investing in research and development to bring new and improved biosimilar products to market. This competition drives innovation and helps to reduce costs, making these treatments more accessible to patients worldwide. Additionally, the availability of multiple dosage forms provides flexibility in treatment options, allowing for more personalized and effective management of autoimmune diseases. The growing demand for these biosimilars is also driven by the increasing prevalence of autoimmune diseases, the need for cost-effective treatment options, and favorable regulatory policies that support the development and approval of biosimilars. Overall, the 80mg, 40mg, and 20mg dosage forms of Adalimumab biosimilars play a vital role in the global market, offering effective and affordable treatment options for patients with autoimmune diseases.
Adult, Child in the Global Adalimumab Biosimilars Market:
The usage of Global Adalimumab Biosimilars Market in adults and children varies significantly due to differences in disease prevalence, treatment protocols, and patient needs. In adults, Adalimumab biosimilars are primarily used to treat chronic autoimmune conditions such as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, Crohn's disease, and ulcerative colitis. These conditions often require long-term treatment to manage symptoms and prevent disease progression. The availability of biosimilars provides a more affordable option for adult patients, reducing the financial burden associated with biologic therapies. Adult patients typically receive the 40mg dosage form, administered via subcutaneous injection every two weeks. In some cases, the 80mg dosage may be used for patients with more severe disease or those who have developed a tolerance to lower doses. The use of Adalimumab biosimilars in adults has been shown to be effective in reducing inflammation, alleviating symptoms, and improving the quality of life for patients with autoimmune diseases. In children, the use of Adalimumab biosimilars is primarily focused on treating juvenile idiopathic arthritis (JIA) and pediatric Crohn's disease. These conditions can significantly impact a child's growth, development, and overall well-being. The availability of biosimilars provides a more affordable treatment option for pediatric patients, ensuring that they have access to effective therapies without imposing a significant financial burden on their families. Pediatric patients typically receive the 20mg dosage form, which is administered via subcutaneous injection. The dosage and frequency of administration may vary based on the child's weight, age, and disease severity. The use of Adalimumab biosimilars in children has been shown to be effective in managing symptoms, reducing inflammation, and improving overall health outcomes. The safety and efficacy of these biosimilars in pediatric patients are supported by rigorous clinical trials and regulatory approvals, ensuring that they meet the same high standards as the original biologic drug. The availability of different dosage forms allows healthcare providers to tailor treatment plans to the specific needs of each child, ensuring optimal efficacy and safety. Overall, the usage of Global Adalimumab Biosimilars Market in adults and children provides effective and affordable treatment options for managing autoimmune diseases, improving patient outcomes, and enhancing the quality of life for patients of all ages.
Global Adalimumab Biosimilars Market Outlook:
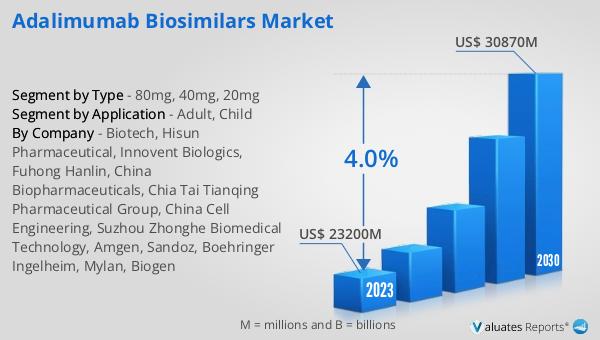
The global Adalimumab Biosimilars market was valued at US$ 23,200 million in 2023 and is anticipated to reach US$ 30,870 million by 2030, witnessing a CAGR of 4.0% during the forecast period 2024-2030. This market outlook indicates a steady growth trajectory driven by the increasing prevalence of autoimmune diseases and the rising demand for cost-effective treatment options. The significant valuation of the market in 2023 underscores the importance of Adalimumab biosimilars in the healthcare industry, providing affordable alternatives to the original biologic drug. The projected growth to US$ 30,870 million by 2030 reflects the ongoing efforts of pharmaceutical companies to develop and market high-quality biosimilar products. The compound annual growth rate (CAGR) of 4.0% during the forecast period highlights the sustained demand for these treatments and the positive impact of favorable regulatory policies that support the development and approval of biosimilars. This market outlook emphasizes the critical role of Adalimumab biosimilars in addressing the needs of patients with autoimmune diseases, offering effective and affordable treatment options that improve patient outcomes and enhance the quality of life.
| Report Metric | Details |
| Report Name | Adalimumab Biosimilars Market |
| Accounted market size in 2023 | US$ 23200 million |
| Forecasted market size in 2030 | US$ 30870 million |
| CAGR | 4.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Biotech, Hisun Pharmaceutical, Innovent Biologics, Fuhong Hanlin, China Biopharmaceuticals, Chia Tai Tianqing Pharmaceutical Group, China Cell Engineering, Suzhou Zhonghe Biomedical Technology, Amgen, Sandoz, Boehringer Ingelheim, Mylan, Biogen |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
