What is Global Small Molecule Chemical Drug CDMO Market?
The Global Small Molecule Chemical Drug CDMO Market refers to the Contract Development and Manufacturing Organizations (CDMOs) that specialize in the development and production of small molecule chemical drugs. These organizations provide a range of services, including drug discovery, process development, and commercial manufacturing, to pharmaceutical companies. Small molecule drugs are typically low molecular weight compounds that can easily enter cells and affect biological processes. CDMOs play a crucial role in the pharmaceutical industry by offering expertise and resources that may not be available in-house for many companies. This allows pharmaceutical companies to focus on their core competencies, such as research and marketing, while outsourcing the complex and resource-intensive processes of drug development and manufacturing. The global market for these services is growing rapidly, driven by increasing demand for new drugs, the complexity of drug development, and the need for cost-effective manufacturing solutions.
Human Medicine, Veterinary Medicine in the Global Small Molecule Chemical Drug CDMO Market:
Human medicine and veterinary medicine are two significant sectors within the Global Small Molecule Chemical Drug CDMO Market. In human medicine, CDMOs are involved in the development and manufacturing of a wide range of therapeutic drugs used to treat various diseases and medical conditions. These include antibiotics, antivirals, anticancer drugs, and medications for chronic conditions such as diabetes and hypertension. The development process for human medicines involves rigorous testing and regulatory approval to ensure safety and efficacy. CDMOs provide the necessary expertise in formulation development, clinical trial manufacturing, and large-scale production to meet these stringent requirements. In veterinary medicine, CDMOs develop and manufacture drugs used to treat diseases in animals. This includes medications for livestock, pets, and other animals. Veterinary drugs must also undergo rigorous testing and regulatory approval, but the requirements can differ from those for human medicines. CDMOs in this sector offer specialized knowledge in veterinary pharmacology and toxicology, as well as the ability to produce drugs in various forms, such as tablets, injectables, and feed additives. The collaboration between pharmaceutical companies and CDMOs in both human and veterinary medicine is essential for bringing new and effective treatments to market. By leveraging the expertise and resources of CDMOs, pharmaceutical companies can accelerate the development process, reduce costs, and ensure high-quality production standards.
Clinical Stage Projects, Commercialization Stage Projects in the Global Small Molecule Chemical Drug CDMO Market:
The Global Small Molecule Chemical Drug CDMO Market is extensively utilized in both clinical stage projects and commercialization stage projects. In clinical stage projects, CDMOs play a pivotal role in the early phases of drug development. This includes preclinical studies, where the safety and efficacy of a drug are tested in laboratory settings and animal models. CDMOs provide the necessary infrastructure and expertise to conduct these studies, including advanced analytical techniques and state-of-the-art laboratory facilities. As the drug progresses to clinical trials, CDMOs are responsible for producing the drug in compliance with Good Manufacturing Practices (GMP) to ensure it is safe for human testing. This involves scaling up the production process from laboratory scale to pilot scale, optimizing the formulation, and ensuring the drug meets all regulatory requirements. In commercialization stage projects, CDMOs support the transition from clinical development to full-scale commercial production. This involves further scaling up the manufacturing process to produce large quantities of the drug, implementing robust quality control measures, and ensuring a consistent supply chain. CDMOs also assist with regulatory submissions and approvals, providing the necessary documentation and data to demonstrate the drug's safety, efficacy, and quality. By partnering with CDMOs, pharmaceutical companies can streamline the development process, reduce time to market, and focus on their core competencies, such as research and marketing. The expertise and resources provided by CDMOs are essential for the successful development and commercialization of new drugs, ultimately benefiting patients and healthcare providers.
Global Small Molecule Chemical Drug CDMO Market Outlook:
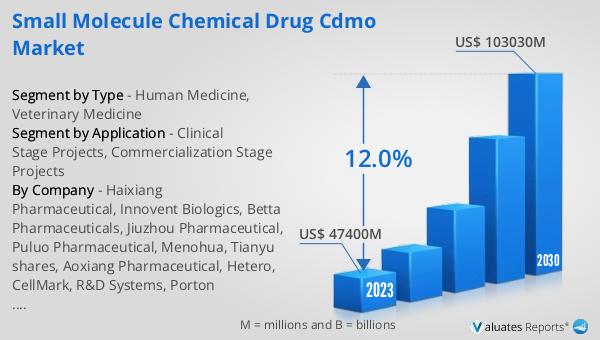
The global Small Molecule Chemical Drug CDMO market was valued at US$ 47,400 million in 2023 and is anticipated to reach US$ 103,030 million by 2030, witnessing a CAGR of 12.0% during the forecast period 2024-2030. This significant growth reflects the increasing demand for CDMO services in the pharmaceutical industry. The rising complexity of drug development, coupled with the need for cost-effective manufacturing solutions, is driving pharmaceutical companies to outsource these processes to specialized CDMOs. By leveraging the expertise and resources of CDMOs, pharmaceutical companies can accelerate the development and commercialization of new drugs, ultimately benefiting patients and healthcare providers. The projected growth of the market underscores the critical role that CDMOs play in the pharmaceutical industry, providing essential services that enable the development and production of innovative therapies.
| Report Metric | Details |
| Report Name | Small Molecule Chemical Drug CDMO Market |
| Accounted market size in 2023 | US$ 47400 million |
| Forecasted market size in 2030 | US$ 103030 million |
| CAGR | 12.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Haixiang Pharmaceutical, Innovent Biologics, Betta Pharmaceuticals, Jiuzhou Pharmaceutical, Puluo Pharmaceutical, Menohua, Tianyu shares, Aoxiang Pharmaceutical, Hetero, CellMark, R&D Systems, Porton Pharmaceuticals, Kai Laiying, Smart Life, Notai Biotech, Aurobindo Pharma, Lonza Group, Catalent Inc., Recipharm AB, Jubilant Pharmova Ltd, Patheon Inc. (Thermo Fisher Scientific Inc.), Boehringer Ingelheim Group, Pfizer CentreSource, Baxter International Inc. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |