What is Global Cell Therapy and Gene Therapy CDMO Market?
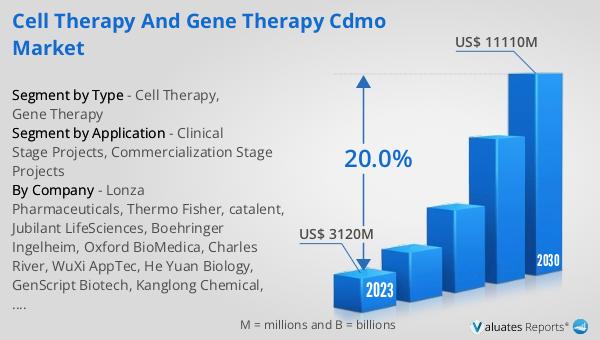
The global Cell Therapy and Gene Therapy CDMO market is a rapidly evolving sector that focuses on providing contract development and manufacturing services for cell and gene therapies. CDMOs, or Contract Development and Manufacturing Organizations, play a crucial role in this market by offering specialized services that range from early-stage development to commercial-scale production. These therapies are at the forefront of medical innovation, aiming to treat, and potentially cure, a wide array of diseases by modifying or replacing defective cells and genes. The market was valued at US$ 3120 million in 2023 and is expected to reach US$ 11110 million by 2030, growing at a compound annual growth rate (CAGR) of 20.0% during the forecast period from 2024 to 2030. This significant growth is driven by increasing investments in biotechnology, advancements in medical research, and a growing number of clinical trials focused on cell and gene therapies. The market's expansion is also fueled by the rising prevalence of chronic diseases and genetic disorders, which necessitate innovative treatment solutions. As a result, the demand for CDMO services in this sector is expected to surge, making it a pivotal area of focus for pharmaceutical and biotechnology companies worldwide.
Cell Therapy, Gene Therapy in the Global Cell Therapy and Gene Therapy CDMO Market:
Cell therapy and gene therapy are two groundbreaking approaches in the field of medical science, particularly within the Global Cell Therapy and Gene Therapy CDMO market. Cell therapy involves the administration of living cells into a patient’s body to treat or cure diseases. These cells can be derived from the patient (autologous) or from a donor (allogeneic). The primary goal is to repair or replace damaged tissues and cells, thereby restoring normal function. Common applications include treatments for cancer, autoimmune diseases, and regenerative medicine. Gene therapy, on the other hand, focuses on modifying or manipulating genes within a patient’s cells to treat or prevent disease. This can involve replacing a faulty gene with a healthy one, inactivating a malfunctioning gene, or introducing a new gene to help fight a disease. Both therapies are highly personalized and require sophisticated technologies and processes for development and manufacturing. CDMOs in this market provide essential services such as cell line development, vector production, process development, and clinical and commercial manufacturing. They also offer regulatory support to navigate the complex approval processes required for these advanced therapies. The integration of cell and gene therapies has the potential to revolutionize the treatment landscape for numerous conditions, offering hope for cures where traditional therapies have failed. The collaboration between biotech companies and CDMOs is crucial for advancing these therapies from the laboratory to the clinic and eventually to the market. This partnership ensures that the therapies are produced at the highest quality standards, meeting stringent regulatory requirements. As the field continues to evolve, the role of CDMOs will become increasingly important in scaling up production and making these innovative treatments accessible to patients worldwide.
Clinical Stage Projects, Commercialization Stage Projects in the Global Cell Therapy and Gene Therapy CDMO Market:
The Global Cell Therapy and Gene Therapy CDMO market plays a vital role in both clinical stage projects and commercialization stage projects. In the clinical stage, CDMOs provide critical support for the development and testing of new therapies. This includes cell line development, vector production, and process optimization to ensure that the therapies are safe and effective for human use. CDMOs also assist with the design and execution of clinical trials, providing the necessary infrastructure and expertise to manage complex regulatory requirements. This support is essential for advancing therapies from preclinical studies to human trials, where they can be tested for safety and efficacy. In the commercialization stage, CDMOs help scale up production to meet market demand. This involves transitioning from small-scale manufacturing used in clinical trials to large-scale production required for commercial distribution. CDMOs offer expertise in process development, quality control, and regulatory compliance to ensure that the therapies are produced consistently and meet all regulatory standards. They also provide logistical support for distribution, ensuring that the therapies reach patients in a timely and efficient manner. The collaboration between biotech companies and CDMOs is crucial for the successful commercialization of cell and gene therapies. By leveraging the specialized expertise and infrastructure of CDMOs, biotech companies can focus on their core competencies of research and development, while ensuring that their therapies are produced and distributed at the highest quality standards. This partnership is essential for bringing innovative treatments to market and making them accessible to patients worldwide. As the field of cell and gene therapy continues to grow, the role of CDMOs will become increasingly important in supporting the development and commercialization of these groundbreaking therapies.
Global Cell Therapy and Gene Therapy CDMO Market Outlook:
The global Cell Therapy and Gene Therapy CDMO market was valued at US$ 3120 million in 2023 and is anticipated to reach US$ 11110 million by 2030, witnessing a CAGR of 20.0% during the forecast period from 2024 to 2030. This remarkable growth underscores the increasing demand for advanced therapeutic solutions and the critical role that CDMOs play in the development and manufacturing of cell and gene therapies. The market's expansion is driven by several factors, including advancements in biotechnology, increased investment in medical research, and a growing number of clinical trials focused on these innovative therapies. Additionally, the rising prevalence of chronic diseases and genetic disorders has created a pressing need for new treatment options, further fueling the demand for CDMO services. As the market continues to evolve, CDMOs will play an increasingly important role in ensuring that cell and gene therapies are developed, manufactured, and distributed at the highest quality standards. This will enable biotech companies to bring their groundbreaking treatments to market more efficiently and effectively, ultimately improving patient outcomes and transforming the landscape of modern medicine.
| Report Metric | Details |
| Report Name | Cell Therapy and Gene Therapy CDMO Market |
| Accounted market size in 2023 | US$ 3120 million |
| Forecasted market size in 2030 | US$ 11110 million |
| CAGR | 20.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Lonza Pharmaceuticals, Thermo Fisher, catalent, Jubilant LifeSciences, Boehringer Ingelheim, Oxford BioMedica, Charles River, WuXi AppTec, He Yuan Biology, GenScript Biotech, Kanglong Chemical, Porton shares, Sano Biologics, Yunzhou Biology |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |