What is Global Rizatriptan Benzoate API Market?
The Global Rizatriptan Benzoate API Market is a specialized segment within the pharmaceutical industry, focusing on the production and distribution of the active pharmaceutical ingredient (API) used in the formulation of medications for treating migraines. Rizatriptan Benzoate is a selective serotonin receptor agonist, commonly known as a triptan, which helps alleviate migraine symptoms by narrowing blood vessels around the brain and reducing substances in the body that can trigger headache pain, nausea, and sensitivity to light and sound. The market for this API is driven by the increasing prevalence of migraines worldwide, which affects millions of people and significantly impacts their quality of life. Pharmaceutical companies are investing in research and development to enhance the efficacy and safety of Rizatriptan Benzoate, aiming to meet the growing demand for effective migraine treatments. The market is characterized by a competitive landscape with several key players involved in the production and supply of this API, striving to maintain high standards of quality and compliance with regulatory requirements. As the demand for migraine medications continues to rise, the Global Rizatriptan Benzoate API Market is expected to expand, offering opportunities for innovation and growth in the pharmaceutical sector.
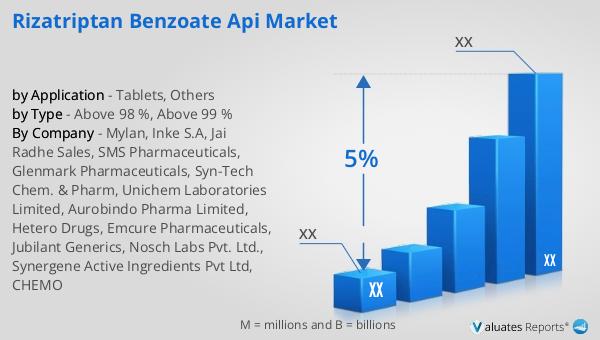
Above 98 %, Above 99 % in the Global Rizatriptan Benzoate API Market:
In the Global Rizatriptan Benzoate API Market, the purity levels of the active pharmaceutical ingredient play a crucial role in determining the quality and efficacy of the final product. Two common purity levels are Above 98% and Above 99%, which refer to the concentration of Rizatriptan Benzoate in the API. The purity level is a critical factor because it directly impacts the effectiveness of the medication and its safety profile. A higher purity level, such as Above 99%, indicates a more refined product with fewer impurities, which can lead to better therapeutic outcomes and reduced risk of adverse effects. Pharmaceutical companies prioritize achieving high purity levels to ensure that their products meet stringent regulatory standards and provide maximum benefit to patients. The process of achieving these purity levels involves advanced manufacturing techniques and rigorous quality control measures. Companies invest in state-of-the-art facilities and employ skilled professionals to oversee the production process, ensuring that the API meets the required specifications. The choice between Above 98% and Above 99% purity levels may also depend on the specific formulation requirements and the intended use of the medication. For instance, certain formulations may require a higher purity level to achieve the desired therapeutic effect, while others may be effective with a slightly lower purity level. The decision is often guided by extensive research and clinical trials that assess the efficacy and safety of different purity levels. Additionally, the cost of production can vary based on the purity level, with higher purity APIs typically being more expensive to produce due to the additional processing and quality control measures required. This cost factor can influence the pricing of the final product and its accessibility to patients. Pharmaceutical companies must balance the need for high purity levels with cost considerations to ensure that their products are both effective and affordable. The competitive nature of the Global Rizatriptan Benzoate API Market drives companies to continuously improve their manufacturing processes and invest in research and development to achieve higher purity levels. This focus on quality and innovation helps companies differentiate their products in the market and build a reputation for reliability and excellence. As the demand for effective migraine treatments continues to grow, the importance of high purity levels in the Rizatriptan Benzoate API Market is expected to remain a key focus for pharmaceutical companies, driving advancements in manufacturing technology and quality assurance practices.
Tablets, Others in the Global Rizatriptan Benzoate API Market:
The Global Rizatriptan Benzoate API Market finds its primary application in the production of tablets, which are the most common form of medication for treating migraines. Tablets offer a convenient and effective way for patients to manage their symptoms, providing a precise dosage of the active ingredient in a form that is easy to administer. The formulation of Rizatriptan Benzoate tablets involves the careful selection of excipients and other ingredients to ensure optimal absorption and efficacy. Pharmaceutical companies invest in research and development to enhance the bioavailability of the active ingredient, ensuring that it is rapidly absorbed into the bloodstream to provide quick relief from migraine symptoms. The production of Rizatriptan Benzoate tablets requires adherence to strict regulatory standards to ensure the safety and efficacy of the final product. Manufacturers must conduct extensive testing and quality control measures to verify that each batch of tablets meets the required specifications. This includes testing for purity, potency, and stability, as well as conducting clinical trials to assess the therapeutic effectiveness of the medication. In addition to tablets, the Global Rizatriptan Benzoate API Market also supports the development of other dosage forms, such as orally disintegrating tablets and nasal sprays. These alternative formulations offer additional options for patients who may have difficulty swallowing traditional tablets or require faster onset of action. Orally disintegrating tablets dissolve quickly in the mouth, providing a convenient option for patients who need rapid relief from migraine symptoms. Nasal sprays, on the other hand, offer an alternative route of administration that can be particularly beneficial for patients experiencing nausea or vomiting during a migraine attack. The development of these alternative dosage forms requires specialized manufacturing processes and expertise to ensure that the active ingredient is delivered effectively and safely. Pharmaceutical companies must invest in research and development to optimize the formulation and delivery of these products, ensuring that they meet the needs of patients and provide effective relief from migraine symptoms. The Global Rizatriptan Benzoate API Market continues to evolve as pharmaceutical companies explore new ways to improve the delivery and efficacy of migraine medications. This includes the development of novel drug delivery systems and the exploration of combination therapies that incorporate Rizatriptan Benzoate with other active ingredients to enhance therapeutic outcomes. As the market for migraine treatments continues to grow, the demand for innovative and effective dosage forms is expected to drive further advancements in the Global Rizatriptan Benzoate API Market, offering new opportunities for pharmaceutical companies to meet the needs of patients and improve their quality of life.
Global Rizatriptan Benzoate API Market Outlook:
The outlook for the Global Rizatriptan Benzoate API Market can be contextualized within the broader pharmaceutical industry landscape. In 2022, the global pharmaceutical market was valued at approximately 1,475 billion USD, with an anticipated compound annual growth rate (CAGR) of 5% over the next six years. This growth is indicative of the increasing demand for pharmaceutical products worldwide, driven by factors such as the rising prevalence of chronic diseases, advancements in drug development, and expanding access to healthcare services. Within this context, the chemical drug market, which includes APIs like Rizatriptan Benzoate, has also shown significant growth. From 2018 to 2022, the chemical drug market is estimated to have increased from 1,005 billion USD to 1,094 billion USD. This growth reflects the ongoing demand for effective and innovative chemical-based therapies, including those for managing migraines. The Global Rizatriptan Benzoate API Market is poised to benefit from these broader industry trends, as the demand for migraine treatments continues to rise. Pharmaceutical companies are likely to invest in research and development to enhance the efficacy and safety of Rizatriptan Benzoate, while also exploring new formulations and delivery methods to meet the diverse needs of patients. As the market for migraine medications expands, the Global Rizatriptan Benzoate API Market is expected to play a crucial role in providing the active ingredients necessary for the development of effective treatments, contributing to the overall growth and innovation within the pharmaceutical industry.
| Report Metric | Details |
| Report Name | Rizatriptan Benzoate API Market |
| CAGR | 5% |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Mylan, Inke S.A, Jai Radhe Sales, SMS Pharmaceuticals, Glenmark Pharmaceuticals, Syn-Tech Chem. & Pharm, Unichem Laboratories Limited, Aurobindo Pharma Limited, Hetero Drugs, Emcure Pharmaceuticals, Jubilant Generics, Nosch Labs Pvt. Ltd., Synergene Active Ingredients Pvt Ltd, CHEMO |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |