What is Global Enoxaparin API Market?
The Global Enoxaparin API Market refers to the worldwide industry focused on the production and distribution of Enoxaparin, an anticoagulant medication primarily used to prevent and treat deep vein thrombosis and pulmonary embolism. Enoxaparin is a low molecular weight heparin (LMWH) that works by inhibiting certain factors in the blood clotting process, making it a crucial component in the management of various cardiovascular conditions. The market for Enoxaparin API is driven by the increasing prevalence of cardiovascular diseases, the rising geriatric population, and the growing demand for effective anticoagulant therapies. Additionally, advancements in pharmaceutical manufacturing processes and the expansion of healthcare infrastructure in emerging economies contribute to the market's growth. The market is characterized by the presence of several key players who are engaged in research and development activities to enhance the efficacy and safety of Enoxaparin formulations. As the demand for anticoagulant therapies continues to rise, the Global Enoxaparin API Market is expected to witness significant growth, offering numerous opportunities for stakeholders in the pharmaceutical industry.
Above 100 IU/mg, Below 100 IU/mg in the Global Enoxaparin API Market:
In the Global Enoxaparin API Market, the differentiation between products above 100 IU/mg and below 100 IU/mg is significant, as it pertains to the potency and concentration of the active pharmaceutical ingredient (API) in the formulations. Enoxaparin products with a concentration above 100 IU/mg are typically used in scenarios where a higher dosage of anticoagulation is required. These formulations are often prescribed for patients with more severe conditions or those who require a more aggressive approach to prevent clot formation. The higher concentration allows for a more potent anticoagulant effect, which is crucial in managing acute cases of deep vein thrombosis or pulmonary embolism. On the other hand, Enoxaparin products with a concentration below 100 IU/mg are generally used for patients who require a milder anticoagulant effect. These formulations are suitable for individuals who are at a lower risk of clot formation or those who need long-term anticoagulation therapy with a reduced risk of bleeding complications. The choice between above 100 IU/mg and below 100 IU/mg formulations is often guided by the patient's medical history, the severity of their condition, and the physician's assessment of the appropriate level of anticoagulation needed. The availability of these different concentrations allows healthcare providers to tailor treatment plans to the specific needs of their patients, ensuring optimal therapeutic outcomes. Furthermore, the production and distribution of Enoxaparin API in these varying concentrations require stringent quality control measures to ensure the safety and efficacy of the final product. Manufacturers must adhere to rigorous regulatory standards and employ advanced manufacturing techniques to maintain the integrity of the API throughout the production process. This involves careful monitoring of the raw materials, precise formulation processes, and thorough testing of the final product to ensure it meets the required specifications. The ability to produce Enoxaparin API in both above and below 100 IU/mg concentrations also reflects the advancements in pharmaceutical technology and the industry's commitment to meeting the diverse needs of patients worldwide. As the demand for anticoagulant therapies continues to grow, the Global Enoxaparin API Market is poised to expand, driven by the increasing prevalence of cardiovascular diseases and the ongoing efforts of pharmaceutical companies to innovate and improve their product offerings.
0.2ml:2000IU, 0.4ml:4000IU, 0.6ml:6000IU, 0.8ml:8000IU, 1ml:10000IU, Others in the Global Enoxaparin API Market:
The usage of Global Enoxaparin API Market in various dosage forms such as 0.2ml:2000IU, 0.4ml:4000IU, 0.6ml:6000IU, 0.8ml:8000IU, 1ml:10000IU, and others, highlights the versatility and adaptability of this anticoagulant medication in addressing different patient needs. Each dosage form is designed to deliver a specific amount of Enoxaparin, allowing healthcare providers to customize treatment plans based on the individual requirements of their patients. The 0.2ml:2000IU dosage is typically used for patients who require a lower dose of anticoagulation, such as those with a lower risk of clot formation or those who need prophylactic treatment to prevent deep vein thrombosis during periods of immobility. This dosage form is also suitable for patients with renal impairment, where a reduced dose is necessary to minimize the risk of bleeding complications. The 0.4ml:4000IU and 0.6ml:6000IU dosages are commonly used for patients who require a moderate level of anticoagulation, such as those undergoing surgery or those with a moderate risk of thromboembolic events. These dosages provide an effective balance between anticoagulation and safety, ensuring that patients receive adequate protection against clot formation without an increased risk of bleeding. The 0.8ml:8000IU and 1ml:10000IU dosages are reserved for patients who require a higher level of anticoagulation, such as those with acute deep vein thrombosis or pulmonary embolism. These higher dosages are also used in patients with a high risk of recurrent thromboembolic events, where aggressive anticoagulation is necessary to prevent further complications. The availability of these various dosage forms allows healthcare providers to tailor treatment plans to the specific needs of their patients, ensuring optimal therapeutic outcomes. Additionally, the production of Enoxaparin API in these different dosages requires stringent quality control measures to ensure the safety and efficacy of the final product. Manufacturers must adhere to rigorous regulatory standards and employ advanced manufacturing techniques to maintain the integrity of the API throughout the production process. This involves careful monitoring of the raw materials, precise formulation processes, and thorough testing of the final product to ensure it meets the required specifications. The ability to produce Enoxaparin API in these various dosages also reflects the advancements in pharmaceutical technology and the industry's commitment to meeting the diverse needs of patients worldwide. As the demand for anticoagulant therapies continues to grow, the Global Enoxaparin API Market is poised to expand, driven by the increasing prevalence of cardiovascular diseases and the ongoing efforts of pharmaceutical companies to innovate and improve their product offerings.
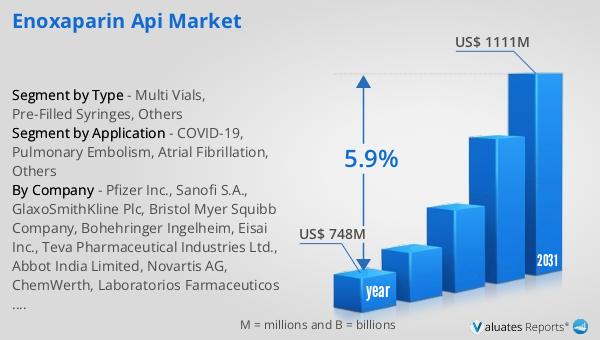
Global Enoxaparin API Market Outlook:
The global market for Enoxaparin API was valued at $171 million in 2024, and it is anticipated to grow significantly, reaching an estimated size of $287 million by 2031. This growth trajectory represents a compound annual growth rate (CAGR) of 5.4% over the forecast period. The increasing demand for Enoxaparin API is driven by several factors, including the rising prevalence of cardiovascular diseases, the aging population, and the growing need for effective anticoagulant therapies. As healthcare systems worldwide continue to expand and improve, the demand for advanced pharmaceutical products like Enoxaparin is expected to rise. The market's growth is also supported by ongoing research and development efforts aimed at enhancing the efficacy and safety of Enoxaparin formulations. Pharmaceutical companies are investing in innovative manufacturing processes and exploring new applications for Enoxaparin to meet the evolving needs of patients and healthcare providers. Additionally, the expansion of healthcare infrastructure in emerging economies is creating new opportunities for market growth, as more patients gain access to advanced medical treatments. As a result, the Global Enoxaparin API Market is poised for significant expansion, offering numerous opportunities for stakeholders in the pharmaceutical industry.
| Report Metric | Details |
| Report Name | Enoxaparin API Market |
| Accounted market size in year | US$ 171 million |
| Forecasted market size in 2031 | US$ 287 million |
| CAGR | 5.4% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Hepalink, Yantai Dongcheng Pharmaceutical, Changzhou Qianhong Biopharma, Hebei Changshan Biochemical Pharmaceutical, Hubei Enoray Biopharmaceutical (Tianjin Chasesun Pharmaceutical), Chongqing Yino Pharma, Chengdu Baiyu Pharmaceutical, Haike Group, Jiangxi Haoran Bio-Pharma, Shandong Chenlong Pharmaceutical (Cisen Pharmaceutical), Stanex |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
