What is Global Sterility Testing Isolator Market?
The Global Sterility Testing Isolator Market is a specialized segment within the broader pharmaceutical and biotechnology industries, focusing on providing controlled environments for sterility testing. Sterility testing is a critical process in ensuring that pharmaceutical products, medical devices, and other healthcare-related items are free from viable contaminating microorganisms. Isolators are used to create a sterile environment, minimizing the risk of contamination during testing. These isolators are designed to provide a barrier between the testing environment and the external environment, ensuring that the samples remain uncontaminated. The market for sterility testing isolators is driven by the increasing demand for safe and effective pharmaceutical products, stringent regulatory requirements, and the need for advanced testing solutions. As the pharmaceutical and biotechnology industries continue to grow, the demand for reliable and efficient sterility testing solutions is expected to rise, further propelling the market. The market is characterized by technological advancements, with manufacturers focusing on developing isolators that offer enhanced performance, ease of use, and compliance with regulatory standards. Overall, the Global Sterility Testing Isolator Market plays a crucial role in ensuring the safety and efficacy of healthcare products.
Single-Chamber Isolator, Multi-Chamber Isolator in the Global Sterility Testing Isolator Market:
Single-Chamber Isolators and Multi-Chamber Isolators are two primary types of isolators used in the Global Sterility Testing Isolator Market, each serving distinct purposes and offering unique advantages. Single-Chamber Isolators are designed with a single, enclosed space where sterility testing is conducted. These isolators are typically used for smaller-scale operations or in settings where space is limited. They provide a controlled environment that is easy to manage and maintain, making them ideal for laboratories and facilities with specific testing needs. Single-Chamber Isolators are often favored for their simplicity and cost-effectiveness, as they require less space and fewer resources to operate. They are particularly useful in situations where the testing volume is low, and the risk of cross-contamination is minimal. On the other hand, Multi-Chamber Isolators consist of multiple interconnected chambers, each serving a specific function within the sterility testing process. These isolators are designed for larger-scale operations and are often used in pharmaceutical manufacturing facilities where high volumes of testing are required. Multi-Chamber Isolators offer greater flexibility and efficiency, as they allow for simultaneous testing of multiple samples or processes. Each chamber can be dedicated to a specific task, such as sample preparation, testing, or storage, reducing the risk of cross-contamination and improving workflow efficiency. The design of Multi-Chamber Isolators also allows for better segregation of different testing processes, ensuring that each step is conducted in a controlled environment. This is particularly important in pharmaceutical and biotechnological applications, where maintaining sterility is critical to product safety and efficacy. Both Single-Chamber and Multi-Chamber Isolators are equipped with advanced features to ensure optimal performance. These may include HEPA filtration systems, automated control systems, and ergonomic designs to enhance user comfort and efficiency. Manufacturers are continually innovating to improve the functionality and reliability of these isolators, incorporating new technologies and materials to meet the evolving needs of the market. The choice between Single-Chamber and Multi-Chamber Isolators depends on various factors, including the scale of operations, available space, budget, and specific testing requirements. While Single-Chamber Isolators are suitable for smaller operations with limited testing needs, Multi-Chamber Isolators are ideal for larger facilities with high testing volumes and complex processes. Both types of isolators play a vital role in ensuring the integrity of sterility testing, contributing to the overall safety and quality of pharmaceutical and biotechnological products. As the demand for advanced sterility testing solutions continues to grow, the market for both Single-Chamber and Multi-Chamber Isolators is expected to expand, driven by technological advancements and increasing regulatory requirements.
Hospitals and Diagnostics Labs, Pharmaceutical and Biotechnological, Others in the Global Sterility Testing Isolator Market:
The Global Sterility Testing Isolator Market finds extensive usage across various sectors, including Hospitals and Diagnostic Labs, Pharmaceutical and Biotechnological industries, and other related fields. In hospitals and diagnostic labs, sterility testing isolators are crucial for ensuring the safety and efficacy of medical products and procedures. These isolators provide a controlled environment for testing samples, reducing the risk of contamination and ensuring accurate results. In hospitals, sterility testing is essential for maintaining the safety of surgical instruments, implants, and other medical devices. Diagnostic labs rely on sterility testing isolators to ensure the accuracy and reliability of test results, which is critical for patient diagnosis and treatment. The Pharmaceutical and Biotechnological industries are major users of sterility testing isolators, as these sectors require stringent quality control measures to ensure the safety and efficacy of their products. Sterility testing is a critical step in the production of pharmaceuticals and biotechnological products, as it ensures that the final products are free from harmful microorganisms. Isolators provide a sterile environment for testing, minimizing the risk of contamination and ensuring compliance with regulatory standards. In pharmaceutical manufacturing, sterility testing isolators are used to test raw materials, in-process samples, and finished products, ensuring that they meet the required safety and quality standards. Biotechnological companies use sterility testing isolators to test biological products, such as vaccines and biologics, which require a high level of sterility to ensure their safety and efficacy. Other sectors that utilize sterility testing isolators include research and development laboratories, contract research organizations, and academic institutions. These organizations rely on sterility testing isolators to ensure the accuracy and reliability of their research findings, which is critical for advancing scientific knowledge and developing new products and technologies. In research and development laboratories, sterility testing isolators are used to test new compounds and formulations, ensuring that they are safe and effective before they are brought to market. Contract research organizations use sterility testing isolators to provide testing services to pharmaceutical and biotechnological companies, ensuring that their products meet the required safety and quality standards. Academic institutions use sterility testing isolators to conduct research and training, providing students and researchers with hands-on experience in sterility testing and quality control. Overall, the Global Sterility Testing Isolator Market plays a vital role in ensuring the safety and efficacy of products and procedures across various sectors, contributing to the overall health and well-being of society.
Global Sterility Testing Isolator Market Outlook:
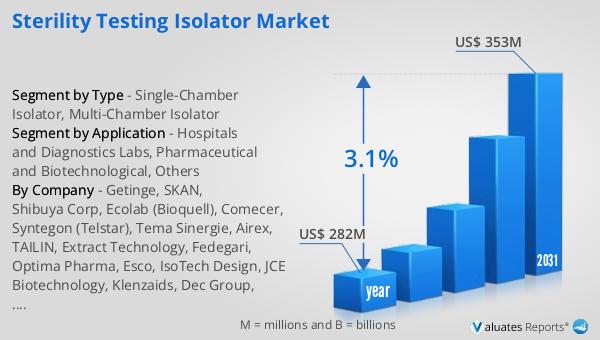
In 2024, the global market for Sterility Testing Isolators was valued at approximately $282 million. Looking ahead, this market is anticipated to grow, reaching an estimated size of $353 million by the year 2031. This growth trajectory represents a compound annual growth rate (CAGR) of 3.1% over the forecast period. The steady increase in market size reflects the rising demand for sterility testing solutions across various industries, including pharmaceuticals, biotechnology, and healthcare. As these industries continue to expand and evolve, the need for reliable and efficient sterility testing isolators becomes increasingly critical. The projected growth in the market is driven by several factors, including technological advancements, increasing regulatory requirements, and the growing emphasis on product safety and quality. Manufacturers are continually innovating to develop isolators that offer enhanced performance, ease of use, and compliance with regulatory standards. This focus on innovation and quality is expected to drive the market forward, ensuring that sterility testing isolators remain an essential component of the pharmaceutical and biotechnological industries. As the market continues to grow, it will play a crucial role in ensuring the safety and efficacy of healthcare products, contributing to the overall health and well-being of society.
| Report Metric | Details |
| Report Name | Sterility Testing Isolator Market |
| Accounted market size in year | US$ 282 million |
| Forecasted market size in 2031 | US$ 353 million |
| CAGR | 3.1% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Getinge, SKAN, Shibuya Corp, Ecolab (Bioquell), Comecer, Syntegon (Telstar), Tema Sinergie, Airex, TAILIN, Extract Technology, Fedegari, Optima Pharma, Esco, IsoTech Design, JCE Biotechnology, Klenzaids, Dec Group, Jacomex, FASTER GmbH, Tofflon |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
