What is Global Custom Sterility Test Isolators Market?
The Global Custom Sterility Test Isolators Market is a specialized segment within the broader pharmaceutical and biotechnology equipment industry. These isolators are designed to provide a controlled environment for sterility testing, ensuring that pharmaceutical products are free from microbial contamination. The market is driven by the increasing demand for safe and effective pharmaceutical products, stringent regulatory requirements, and the need for advanced testing solutions. Custom sterility test isolators are tailored to meet specific testing needs, offering flexibility and precision in various applications. They are essential in maintaining the integrity of sterility tests, which are crucial for ensuring the safety and efficacy of drugs and other medical products. The market is characterized by technological advancements, with manufacturers focusing on developing isolators that offer enhanced performance, ease of use, and compliance with international standards. As the pharmaceutical and biotechnology industries continue to grow, the demand for custom sterility test isolators is expected to rise, driven by the need for reliable and efficient testing solutions. The market is also influenced by factors such as increasing investments in research and development, the expansion of pharmaceutical manufacturing facilities, and the growing emphasis on quality assurance in healthcare.
Single-Chamber Isolator, Multi-Chamber Isolator in the Global Custom Sterility Test Isolators Market:
Single-Chamber Isolators and Multi-Chamber Isolators are two primary types of equipment within the Global Custom Sterility Test Isolators Market, each serving distinct purposes and offering unique advantages. Single-Chamber Isolators are designed to provide a controlled environment within a single, enclosed space. They are typically used for smaller-scale operations where the testing of sterility can be conducted within a confined area. These isolators are favored for their simplicity and ease of use, making them ideal for laboratories and facilities with limited space or resources. The single-chamber design allows for straightforward operation and maintenance, reducing the complexity associated with more extensive systems. They are often employed in settings where the volume of testing is moderate, and the need for flexibility and adaptability is paramount. On the other hand, Multi-Chamber Isolators consist of multiple interconnected chambers, each serving a specific function within the sterility testing process. These isolators are designed for larger-scale operations, where the testing of multiple samples or batches is required simultaneously. The multi-chamber design allows for greater efficiency and throughput, as different stages of the testing process can be conducted concurrently within separate chambers. This design is particularly beneficial in high-volume pharmaceutical manufacturing environments, where time and resource optimization are critical. Multi-Chamber Isolators offer enhanced flexibility, allowing for the customization of each chamber to meet specific testing requirements. This adaptability is crucial in environments where diverse testing protocols are employed, and the need for specialized conditions is essential. Both Single-Chamber and Multi-Chamber Isolators are equipped with advanced features to ensure the integrity of the testing environment. These features include HEPA filtration systems, automated control systems, and robust construction materials that prevent contamination and ensure compliance with regulatory standards. The choice between Single-Chamber and Multi-Chamber Isolators often depends on the specific needs of the facility, the volume of testing required, and the available resources. While Single-Chamber Isolators are ideal for smaller operations with limited space, Multi-Chamber Isolators are better suited for larger facilities with high testing demands. Both types of isolators play a crucial role in the Global Custom Sterility Test Isolators Market, providing essential solutions for maintaining the sterility and safety of pharmaceutical products. As the demand for reliable and efficient sterility testing solutions continues to grow, manufacturers are focusing on developing isolators that offer enhanced performance, ease of use, and compliance with international standards. This focus on innovation and quality assurance is driving the growth of the market, ensuring that pharmaceutical and biotechnology companies have access to the tools they need to meet the highest standards of safety and efficacy.
Hospitals and Diagnostics Labs, Pharmaceutical and Biotechnological, Others in the Global Custom Sterility Test Isolators Market:
The usage of Global Custom Sterility Test Isolators Market spans across various sectors, including Hospitals and Diagnostics Labs, Pharmaceutical and Biotechnological industries, and other related fields. In Hospitals and Diagnostics Labs, sterility test isolators are crucial for ensuring the safety and efficacy of medical products and procedures. These isolators provide a controlled environment for testing the sterility of medical devices, surgical instruments, and other healthcare products. By preventing microbial contamination, they help maintain the highest standards of patient safety and care. In the Pharmaceutical and Biotechnological industries, sterility test isolators are essential for ensuring the quality and safety of drugs and biologics. These isolators are used to test the sterility of raw materials, intermediates, and finished products, ensuring that they are free from harmful microorganisms. The use of isolators in these industries is driven by stringent regulatory requirements and the need for reliable and efficient testing solutions. By providing a controlled environment for sterility testing, these isolators help pharmaceutical and biotechnology companies meet the highest standards of quality and compliance. In addition to Hospitals and Diagnostics Labs, and Pharmaceutical and Biotechnological industries, sterility test isolators are also used in other sectors, such as food and beverage, cosmetics, and research and development. In the food and beverage industry, these isolators are used to test the sterility of food products and packaging materials, ensuring that they are free from harmful microorganisms. In the cosmetics industry, sterility test isolators are used to test the safety and efficacy of cosmetic products, ensuring that they are free from microbial contamination. In research and development, these isolators are used to test the sterility of experimental materials and equipment, ensuring that they are free from contamination and suitable for use in scientific studies. The versatility and adaptability of sterility test isolators make them an essential tool in a wide range of industries, providing reliable and efficient solutions for maintaining the highest standards of safety and quality. As the demand for safe and effective products continues to grow, the usage of sterility test isolators is expected to increase, driven by the need for reliable and efficient testing solutions.
Global Custom Sterility Test Isolators Market Outlook:
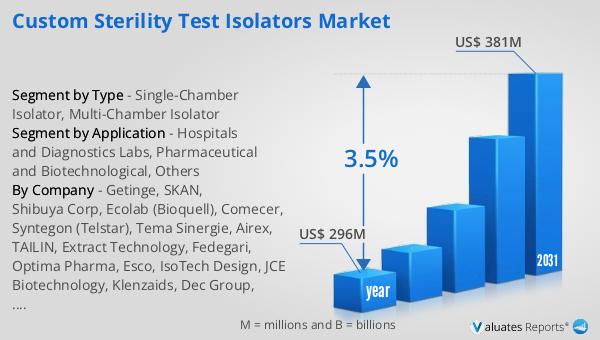
The global market for Custom Sterility Test Isolators was valued at $296 million in 2024 and is anticipated to expand to a revised size of $381 million by 2031, reflecting a compound annual growth rate (CAGR) of 3.5% over the forecast period. This growth trajectory underscores the increasing demand for advanced sterility testing solutions across various industries. The market's expansion is driven by several factors, including the rising need for safe and effective pharmaceutical products, stringent regulatory requirements, and the growing emphasis on quality assurance in healthcare. As pharmaceutical and biotechnology companies continue to invest in research and development, the demand for reliable and efficient sterility test isolators is expected to rise. These isolators play a crucial role in ensuring the safety and efficacy of drugs and other medical products, providing a controlled environment for sterility testing. The market is also influenced by technological advancements, with manufacturers focusing on developing isolators that offer enhanced performance, ease of use, and compliance with international standards. As the market continues to grow, companies are expected to focus on innovation and quality assurance, ensuring that they have access to the tools they need to meet the highest standards of safety and efficacy. This focus on innovation and quality assurance is driving the growth of the market, ensuring that pharmaceutical and biotechnology companies have access to the tools they need to meet the highest standards of safety and efficacy.
| Report Metric | Details |
| Report Name | Custom Sterility Test Isolators Market |
| Accounted market size in year | US$ 296 million |
| Forecasted market size in 2031 | US$ 381 million |
| CAGR | 3.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Getinge, SKAN, Shibuya Corp, Ecolab (Bioquell), Comecer, Syntegon (Telstar), Tema Sinergie, Airex, TAILIN, Extract Technology, Fedegari, Optima Pharma, Esco, IsoTech Design, JCE Biotechnology, Klenzaids, Dec Group, Jacomex, FASTER GmbH, Tofflon |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
