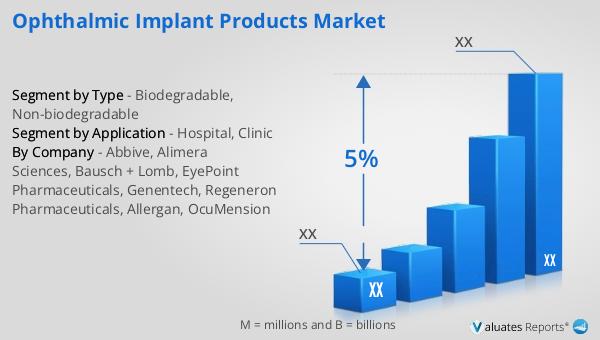
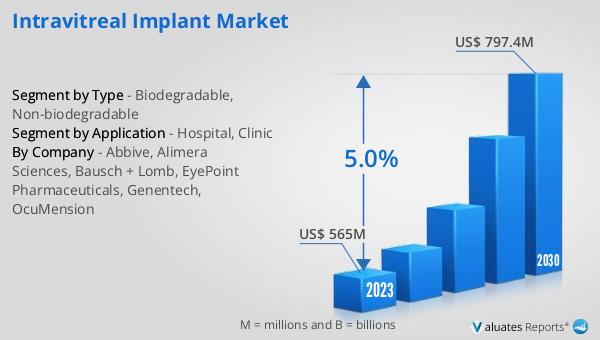
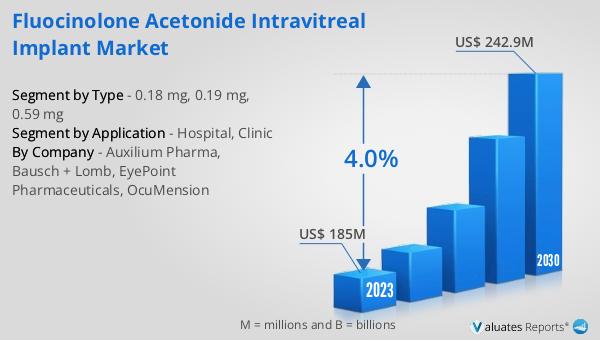
What is Global Non-biodegradable Implantable Drug Delivery System Market?
The Global Non-biodegradable Implantable Drug Delivery System Market refers to the segment of the medical device industry that focuses on implantable devices designed to deliver drugs directly to specific parts of the body over an extended period. Unlike biodegradable systems, these implants do not break down within the body and must be removed or replaced after their drug supply is exhausted. These systems are particularly useful for chronic conditions requiring long-term medication, as they provide a controlled and sustained release of drugs, improving patient compliance and therapeutic outcomes. The market encompasses various types of implants, including those used for delivering hormones, pain management drugs, and treatments for chronic diseases. The non-biodegradable nature of these implants ensures that they remain effective over a longer duration, making them a reliable option for patients and healthcare providers. The market is driven by advancements in medical technology, increasing prevalence of chronic diseases, and the growing demand for targeted drug delivery systems.
Subcutaneous Implant Type, Injection Implant Type, Other in the Global Non-biodegradable Implantable Drug Delivery System Market:
Subcutaneous implants, injection implants, and other types of non-biodegradable implantable drug delivery systems each serve unique roles in the medical field. Subcutaneous implants are placed just under the skin and are designed to release medication slowly over time. These are often used for hormone therapies, such as contraceptives or treatments for hormone-related conditions. The advantage of subcutaneous implants is their ease of insertion and removal, as well as their ability to provide a steady release of medication, which can improve patient adherence to treatment regimens. Injection implants, on the other hand, are typically administered through a syringe and are designed to release medication at a controlled rate. These are often used for pain management, where a steady dose of medication is required over an extended period. Injection implants can be particularly beneficial for patients with chronic pain conditions, as they can reduce the need for frequent dosing and provide more consistent pain relief. Other types of non-biodegradable implantable drug delivery systems include those used for targeted drug delivery to specific organs or tissues. For example, some implants are designed to deliver chemotherapy drugs directly to a tumor site, minimizing the systemic side effects often associated with cancer treatment. These targeted delivery systems can improve the efficacy of treatment by concentrating the drug at the site of action, while reducing the impact on healthy tissues. Additionally, non-biodegradable implants are used in ophthalmology for the treatment of eye conditions such as glaucoma or macular degeneration. These implants can provide a sustained release of medication directly to the eye, improving treatment outcomes and reducing the need for frequent eye drops or injections. In reproductive health, non-biodegradable implants are used for long-term contraception, providing a reliable and reversible method of birth control. These implants release hormones that prevent ovulation, offering an effective alternative to daily contraceptive pills. Overall, the versatility and reliability of non-biodegradable implantable drug delivery systems make them a valuable tool in the management of various medical conditions.
Tumor, Ophthalmology, Reproductive Health, Other in the Global Non-biodegradable Implantable Drug Delivery System Market:
The usage of Global Non-biodegradable Implantable Drug Delivery Systems spans several critical areas, including tumor treatment, ophthalmology, reproductive health, and other medical fields. In the treatment of tumors, these implants are often used to deliver chemotherapy drugs directly to the tumor site. This localized delivery method helps to concentrate the drug where it is needed most, enhancing its effectiveness while minimizing systemic side effects. By targeting the tumor directly, these implants can reduce the overall dosage required, potentially lowering the risk of adverse reactions and improving patient outcomes. In ophthalmology, non-biodegradable implants are used to treat conditions such as glaucoma and macular degeneration. These implants can provide a sustained release of medication directly to the eye, ensuring a consistent therapeutic effect and reducing the need for frequent eye drops or injections. This can be particularly beneficial for patients who have difficulty adhering to regular treatment schedules, as the implant can maintain the necessary drug levels over an extended period. In reproductive health, non-biodegradable implants are commonly used for long-term contraception. These implants release hormones that prevent ovulation, offering a reliable and reversible method of birth control. The convenience of a single implant that can last for several years makes it an attractive option for many women, reducing the need for daily contraceptive pills and improving adherence to contraceptive regimens. Other areas where non-biodegradable implantable drug delivery systems are used include pain management and chronic disease treatment. For patients with chronic pain conditions, these implants can provide a steady release of pain medication, reducing the need for frequent dosing and providing more consistent pain relief. This can improve the quality of life for patients by allowing them to manage their pain more effectively. In the treatment of chronic diseases, such as diabetes or cardiovascular conditions, non-biodegradable implants can deliver medication directly to the affected area, improving the efficacy of treatment and reducing the risk of side effects. Overall, the versatility and reliability of non-biodegradable implantable drug delivery systems make them a valuable tool in the management of various medical conditions, offering targeted and sustained drug delivery that can improve patient outcomes and adherence to treatment regimens.
Global Non-biodegradable Implantable Drug Delivery System Market Outlook:
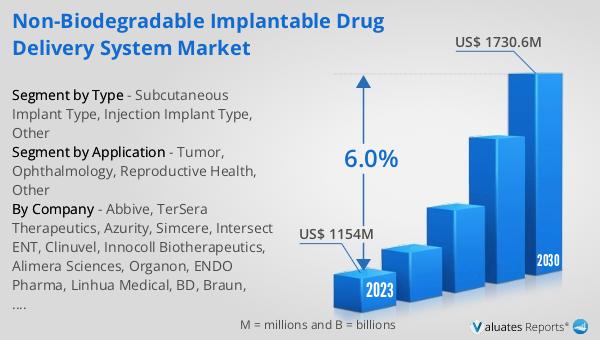
The global Non-biodegradable Implantable Drug Delivery System market was valued at US$ 1154 million in 2023 and is anticipated to reach US$ 1730.6 million by 2030, witnessing a CAGR of 6.0% during the forecast period 2024-2030. According to our research, the global market for medical devices is estimated at US$ 603 billion in the year 2023 and will be growing at a CAGR of 5% during the next six years. These systems are designed to deliver drugs directly to specific parts of the body over an extended period, providing a controlled and sustained release of medication. Unlike biodegradable systems, non-biodegradable implants do not break down within the body and must be removed or replaced after their drug supply is exhausted. This ensures that they remain effective over a longer duration, making them a reliable option for patients and healthcare providers. The market is driven by advancements in medical technology, increasing prevalence of chronic diseases, and the growing demand for targeted drug delivery systems.
| Report Metric | Details |
| Report Name | Non-biodegradable Implantable Drug Delivery System Market |
| Accounted market size in 2023 | US$ 1154 million |
| Forecasted market size in 2030 | US$ 1730.6 million |
| CAGR | 6.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Abbive, TerSera Therapeutics, Azurity, Simcere, Intersect ENT, Clinuvel, Innocoll Biotherapeutics, Alimera Sciences, Organon, ENDO Pharma, Linhua Medical, BD, Braun, AngioDynamics, Smiths Medical, Teleflex, Cook Medical, Fresenius, Vygon, PFM Medical, Districlass |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |