What is Real-World Evidence in Healthcare - Global Market?
Real-World Evidence (RWE) in healthcare refers to the clinical data collected from real-world settings, such as hospitals, clinics, and patient registries, rather than controlled clinical trials. This data provides insights into how treatments work in everyday practice, offering a more comprehensive understanding of their effectiveness and safety. RWE is increasingly important in the global healthcare market as it helps bridge the gap between clinical trials and actual patient outcomes. By analyzing data from diverse sources, including electronic health records, insurance claims, and patient-reported outcomes, healthcare providers and researchers can make more informed decisions. This approach not only enhances patient care but also supports regulatory decision-making and healthcare policy development. As the demand for personalized medicine grows, RWE plays a crucial role in tailoring treatments to individual patient needs, ultimately improving healthcare outcomes worldwide. The integration of RWE into healthcare systems is transforming the industry by providing valuable insights that drive innovation and improve patient care.
Service, Data Set in the Real-World Evidence in Healthcare - Global Market:
In the realm of Real-World Evidence (RWE) in healthcare, services and data sets play a pivotal role in shaping the global market. Services related to RWE encompass a wide range of activities, including data collection, analysis, and interpretation. These services are crucial for healthcare providers, pharmaceutical companies, and regulatory bodies to harness the full potential of RWE. Data collection services involve gathering information from various sources, such as electronic health records, insurance claims, and patient registries. This data is then analyzed to extract meaningful insights that can inform clinical decision-making and policy development. The analysis of RWE data requires sophisticated tools and methodologies to ensure accuracy and reliability. Advanced analytics, machine learning, and artificial intelligence are often employed to process large volumes of data and identify patterns that may not be apparent through traditional methods. Interpretation services involve translating complex data into actionable insights that can guide healthcare strategies and interventions. These services are essential for stakeholders to understand the implications of RWE and make informed decisions. Data sets based on RWE are diverse and encompass a wide range of information, including patient demographics, treatment outcomes, and healthcare utilization patterns. These data sets provide a comprehensive view of how treatments perform in real-world settings, offering valuable insights into their effectiveness and safety. The integration of RWE data sets into healthcare systems enables stakeholders to make evidence-based decisions that improve patient care and outcomes. As the demand for personalized medicine grows, RWE data sets are becoming increasingly important in tailoring treatments to individual patient needs. By leveraging RWE data, healthcare providers can identify the most effective treatments for specific patient populations, ultimately enhancing the quality of care. Furthermore, RWE data sets support regulatory decision-making by providing evidence of treatment effectiveness and safety in diverse patient populations. This evidence is crucial for the approval and reimbursement of new therapies and medical devices. In summary, services and data sets based on Real-World Evidence are integral to the global healthcare market, providing the foundation for informed decision-making and improved patient outcomes.
Drug Development and Approval, Development and Approval of Medical Devices in the Real-World Evidence in Healthcare - Global Market:
The utilization of Real-World Evidence (RWE) in healthcare significantly impacts drug development and approval, as well as the development and approval of medical devices. In drug development, RWE provides valuable insights into how new treatments perform in real-world settings, complementing data from clinical trials. This information is crucial for pharmaceutical companies to understand the effectiveness and safety of their products in diverse patient populations. By analyzing RWE, companies can identify potential side effects, optimize dosing regimens, and tailor treatments to specific patient groups. This approach not only enhances the drug development process but also supports regulatory submissions by providing robust evidence of a drug's real-world performance. Regulatory agencies, such as the FDA and EMA, increasingly rely on RWE to make informed decisions about drug approvals and post-market surveillance. In the realm of medical devices, RWE plays a similar role by providing evidence of device performance in everyday clinical practice. This data is essential for manufacturers to demonstrate the safety and efficacy of their products, supporting regulatory approvals and market access. RWE also aids in the post-market monitoring of medical devices, allowing manufacturers to identify and address potential issues promptly. By leveraging RWE, companies can improve the design and functionality of their devices, ultimately enhancing patient outcomes. Furthermore, RWE supports the development of innovative medical devices by providing insights into unmet clinical needs and guiding the design of new technologies. In summary, the use of Real-World Evidence in drug development and approval, as well as the development and approval of medical devices, is transforming the healthcare industry by providing robust evidence that supports regulatory decision-making and improves patient care.
Real-World Evidence in Healthcare - Global Market Outlook:
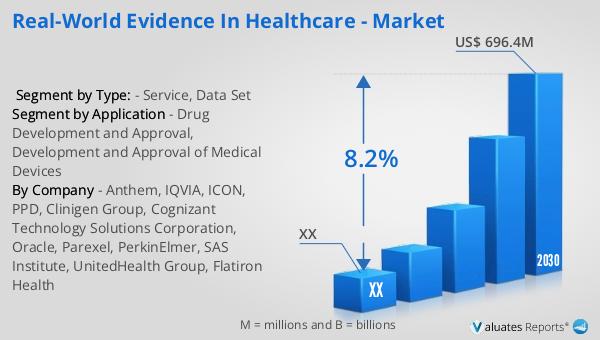
The global market for Real-World Evidence in healthcare was valued at approximately $401 million in 2023. It is projected to grow to a revised size of $696.4 million by 2030, reflecting a compound annual growth rate (CAGR) of 8.2% during the forecast period from 2024 to 2030. This growth underscores the increasing importance of RWE in the healthcare industry, as stakeholders recognize its value in improving patient outcomes and supporting regulatory decision-making. In parallel, the global market for medical devices is estimated to be worth $603 billion in 2023, with a projected CAGR of 5% over the next six years. This growth highlights the expanding role of medical devices in healthcare and the need for robust evidence to support their development and approval. As the demand for personalized medicine and innovative therapies continues to rise, the integration of RWE into healthcare systems is expected to drive further advancements in the industry. By providing valuable insights into treatment effectiveness and safety, RWE is poised to play a critical role in shaping the future of healthcare.
| Report Metric | Details |
| Report Name | Real-World Evidence in Healthcare - Market |
| Forecasted market size in 2030 | US$ 696.4 million |
| CAGR | 8.2% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Anthem, IQVIA, ICON, PPD, Clinigen Group, Cognizant Technology Solutions Corporation, Oracle, Parexel, PerkinElmer, SAS Institute, UnitedHealth Group, Flatiron Health |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |