What is Cardiovascular Medical Device and Diagnostics Trials - Global Market?
Cardiovascular medical device and diagnostics trials are a crucial component of the global healthcare landscape, focusing on the development and evaluation of devices and diagnostic tools used in the treatment and management of cardiovascular diseases. These trials are essential for ensuring the safety and efficacy of new technologies before they are introduced to the market. The global market for these trials is driven by the increasing prevalence of cardiovascular diseases, advancements in medical technology, and the growing demand for minimally invasive procedures. Cardiovascular diseases remain a leading cause of mortality worldwide, necessitating continuous innovation and improvement in medical devices and diagnostics. The trials encompass a wide range of devices, including cardiac rhythm management devices, interventional cardiac devices, and cardiac prosthetic devices, among others. These trials are conducted in various phases, starting from initial feasibility studies to large-scale clinical trials, to gather comprehensive data on the performance and safety of the devices. The insights gained from these trials not only aid in regulatory approvals but also help in refining the design and functionality of the devices, ultimately improving patient outcomes. As the global healthcare industry continues to evolve, the importance of cardiovascular medical device and diagnostics trials is expected to grow, contributing significantly to the advancement of cardiovascular care.
Cardiac Rhythm Management Devices, Interventional Cardiac Devices, Cardiac Prosthetic Devices, Other in the Cardiovascular Medical Device and Diagnostics Trials - Global Market:
Cardiac rhythm management devices are a critical segment within the cardiovascular medical device and diagnostics trials market. These devices, which include pacemakers, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices, are designed to manage and correct abnormal heart rhythms. Pacemakers are used to treat bradycardia, a condition characterized by a slow heart rate, by sending electrical impulses to stimulate the heart to beat at a normal rate. ICDs, on the other hand, are used to prevent sudden cardiac arrest by delivering a shock to the heart when a life-threatening arrhythmia is detected. CRT devices are used to treat heart failure by coordinating the contractions of the heart's ventricles, improving the heart's efficiency. The trials for these devices focus on assessing their safety, effectiveness, and long-term performance in diverse patient populations. Interventional cardiac devices, another significant category, include stents, catheters, and angioplasty balloons, which are used in procedures to open blocked or narrowed coronary arteries. These devices play a crucial role in the treatment of coronary artery disease, a leading cause of heart attacks. The trials for interventional devices evaluate their ability to restore blood flow, reduce symptoms, and improve patient quality of life. Cardiac prosthetic devices, such as artificial heart valves and ventricular assist devices (VADs), are used to replace or support damaged heart structures. These devices are vital for patients with severe heart valve disease or heart failure. The trials for prosthetic devices focus on their durability, biocompatibility, and impact on patient survival and quality of life. Other devices in the cardiovascular medical device and diagnostics trials market include diagnostic imaging tools, such as echocardiography and cardiac MRI, which are used to visualize the heart's structure and function. These tools are essential for accurate diagnosis and treatment planning. The trials for diagnostic devices assess their accuracy, reliability, and ability to provide detailed information about the heart's condition. Overall, the cardiovascular medical device and diagnostics trials market is characterized by continuous innovation and development, driven by the need to address the growing burden of cardiovascular diseases worldwide. These trials are essential for ensuring that new devices and technologies meet the highest standards of safety and efficacy, ultimately improving patient outcomes and quality of life.
Hospitals, Clinics, Other in the Cardiovascular Medical Device and Diagnostics Trials - Global Market:
The usage of cardiovascular medical device and diagnostics trials in hospitals is extensive, as these institutions are often the primary sites for conducting clinical trials. Hospitals provide the necessary infrastructure, resources, and expertise to conduct complex trials, ensuring that the devices are tested in real-world settings. In hospitals, cardiovascular devices are used for a wide range of procedures, from routine diagnostic tests to advanced surgical interventions. For instance, cardiac rhythm management devices are implanted in patients with arrhythmias, while interventional cardiac devices are used in catheterization labs to treat coronary artery disease. Hospitals also play a crucial role in post-market surveillance, monitoring the long-term performance and safety of the devices in patients. Clinics, on the other hand, are often involved in the early stages of cardiovascular medical device and diagnostics trials, particularly in feasibility and pilot studies. These smaller healthcare settings provide a more controlled environment for testing new devices and technologies. Clinics are also instrumental in patient recruitment and follow-up, ensuring that trial participants receive consistent care and monitoring throughout the study. In clinics, cardiovascular devices are used for diagnostic purposes, such as stress tests and echocardiograms, as well as for minor procedures, such as pacemaker implantation. Other settings, such as research institutions and academic medical centers, also play a significant role in cardiovascular medical device and diagnostics trials. These institutions often lead the development of new technologies and conduct pioneering research to advance the field of cardiovascular medicine. They collaborate with hospitals and clinics to conduct multi-center trials, ensuring that the devices are tested across diverse patient populations and healthcare settings. Additionally, these institutions contribute to the training and education of healthcare professionals, ensuring that they are equipped with the knowledge and skills to use the latest cardiovascular devices and technologies. Overall, the usage of cardiovascular medical device and diagnostics trials in hospitals, clinics, and other settings is essential for advancing cardiovascular care and improving patient outcomes. These trials provide valuable data on the safety, efficacy, and performance of new devices, ensuring that they meet the highest standards of quality and reliability. As the global burden of cardiovascular diseases continues to rise, the importance of these trials in developing innovative solutions to address this challenge cannot be overstated.
Cardiovascular Medical Device and Diagnostics Trials - Global Market Outlook:
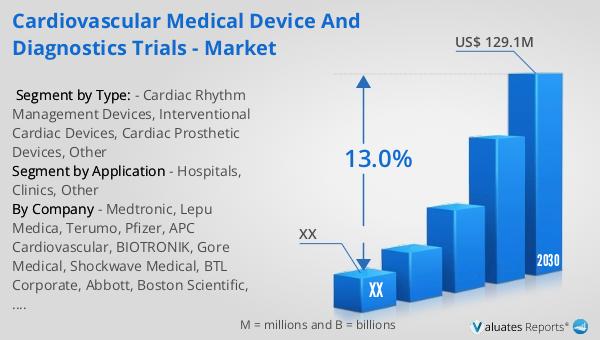
The global market for cardiovascular medical device and diagnostics trials was valued at approximately US$ 55 million in 2023. It is projected to grow significantly, reaching an estimated size of US$ 129.1 million by 2030, with a compound annual growth rate (CAGR) of 13.0% during the forecast period from 2024 to 2030. This growth is indicative of the increasing demand for advanced cardiovascular devices and diagnostics, driven by the rising prevalence of cardiovascular diseases and the need for innovative treatment solutions. According to our research, the broader global market for medical devices is estimated to be worth US$ 603 billion in 2023, with a projected CAGR of 5% over the next six years. This highlights the substantial growth potential within the cardiovascular segment, as advancements in technology and healthcare infrastructure continue to drive the development and adoption of new devices. The cardiovascular medical device and diagnostics trials market is poised for significant expansion, as healthcare providers and patients alike seek more effective and efficient solutions for managing cardiovascular conditions. The increasing focus on personalized medicine and minimally invasive procedures further underscores the importance of these trials in shaping the future of cardiovascular care. As the market continues to evolve, stakeholders across the healthcare ecosystem will need to collaborate and innovate to address the growing demand for high-quality cardiovascular devices and diagnostics.
| Report Metric | Details |
| Report Name | Cardiovascular Medical Device and Diagnostics Trials - Market |
| Forecasted market size in 2030 | US$ 129.1 million |
| CAGR | 13.0% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Medtronic, Lepu Medica, Terumo, Pfizer, APC Cardiovascular, BIOTRONIK, Gore Medical, Shockwave Medical, BTL Corporate, Abbott, Boston Scientific, Edwards Lifesciences, Abbott Laboratories, Johnson & Johnson, Getinge, SynexMed, W. L. Gore & Associate, Lepu Medical Technology, Sorin Group, B.Braun, Tegra, Demax Medical, Newtech Medical Devices, Argon Medical Devices, Eurocor, Merit Medical Systems |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |