What is Global Fluocinolone Acetonide Intravitreal Implant Market?
The Global Fluocinolone Acetonide Intravitreal Implant Market refers to the worldwide market for a specific type of medical implant used to treat eye conditions. Fluocinolone acetonide is a corticosteroid that helps reduce inflammation and is used in the form of an intravitreal implant, which is placed directly into the eye. This implant is particularly effective in treating chronic non-infectious uveitis affecting the posterior segment of the eye and diabetic macular edema. The market encompasses various aspects such as the production, distribution, and sales of these implants. It includes different stakeholders like pharmaceutical companies, healthcare providers, and patients. The market is driven by factors such as the increasing prevalence of eye diseases, advancements in medical technology, and the growing aging population. The demand for effective and long-lasting treatments for eye conditions is also a significant driver. The market is segmented based on different dosages of the implant, including 0.18 mg, 0.19 mg, and 0.59 mg, each catering to specific medical needs and patient conditions. The global reach of this market indicates its importance in the healthcare sector, providing essential treatments to improve the quality of life for patients with severe eye conditions.
0.18 mg, 0.19 mg, 0.59 mg in the Global Fluocinolone Acetonide Intravitreal Implant Market:
The Global Fluocinolone Acetonide Intravitreal Implant Market includes various dosages such as 0.18 mg, 0.19 mg, and 0.59 mg, each designed to meet specific medical requirements. The 0.18 mg dosage is typically used for patients with less severe conditions or those who may require a lower dose due to other health considerations. This dosage provides a steady release of the medication over a prolonged period, ensuring consistent treatment and reducing the need for frequent medical interventions. The 0.19 mg dosage is slightly higher and is often prescribed for patients with moderate eye conditions. It offers a balance between efficacy and safety, providing sufficient medication to manage inflammation and other symptoms effectively. The 0.59 mg dosage is the highest among the three and is used for patients with more severe or chronic conditions. This dosage ensures a higher concentration of the medication, which can be crucial for managing more aggressive forms of eye diseases. Each dosage form is designed to release the medication slowly over time, providing long-term relief and reducing the frequency of injections needed. The choice of dosage depends on various factors, including the severity of the condition, patient health status, and the specific medical needs of the patient. The availability of different dosages allows healthcare providers to tailor treatments to individual patients, ensuring optimal outcomes. The market for these implants is driven by the need for effective, long-lasting treatments for eye conditions, and the availability of multiple dosages enhances the flexibility and effectiveness of treatment options. The implants are designed to be biocompatible and safe, minimizing the risk of adverse reactions and ensuring patient comfort. The development and production of these implants involve advanced medical technology and rigorous testing to ensure their safety and efficacy. The market also includes various stakeholders such as pharmaceutical companies, healthcare providers, and regulatory bodies, all working together to ensure the availability and accessibility of these essential medical devices. The global reach of this market highlights its importance in the healthcare sector, providing critical treatments for patients with severe eye conditions and improving their quality of life.
Hospital, Clinic in the Global Fluocinolone Acetonide Intravitreal Implant Market:
The usage of Global Fluocinolone Acetonide Intravitreal Implants in hospitals and clinics is crucial for the effective management of severe eye conditions. In hospitals, these implants are often used in specialized ophthalmology departments where patients with complex and chronic eye diseases are treated. Hospitals provide a comprehensive range of services, including diagnosis, treatment, and follow-up care, making them ideal settings for the administration of these implants. The implants are typically administered by ophthalmologists who have specialized training in eye care. The hospital setting allows for the use of advanced diagnostic tools and equipment, ensuring accurate diagnosis and effective treatment planning. In clinics, the usage of these implants is also significant, particularly in outpatient settings where patients may not require hospitalization but still need specialized care. Clinics provide a more accessible and convenient option for patients, allowing them to receive treatment without the need for a hospital stay. The administration of the implants in clinics is usually done by ophthalmologists or trained healthcare professionals, ensuring that patients receive high-quality care. The use of these implants in both hospitals and clinics helps to reduce the burden on healthcare systems by providing effective, long-lasting treatments that minimize the need for frequent medical interventions. This is particularly important for patients with chronic conditions who require ongoing management. The implants provide a sustained release of medication, reducing the frequency of injections and improving patient compliance. The availability of different dosages allows healthcare providers to tailor treatments to individual patient needs, ensuring optimal outcomes. The use of these implants in hospitals and clinics also highlights the importance of specialized care in the management of severe eye conditions. The collaboration between healthcare providers, pharmaceutical companies, and regulatory bodies ensures that patients have access to safe and effective treatments. The global reach of this market underscores its significance in the healthcare sector, providing essential treatments that improve the quality of life for patients with severe eye conditions. The use of these implants in hospitals and clinics is a testament to the advancements in medical technology and the ongoing efforts to provide effective treatments for complex and chronic diseases.
Global Fluocinolone Acetonide Intravitreal Implant Market Outlook:
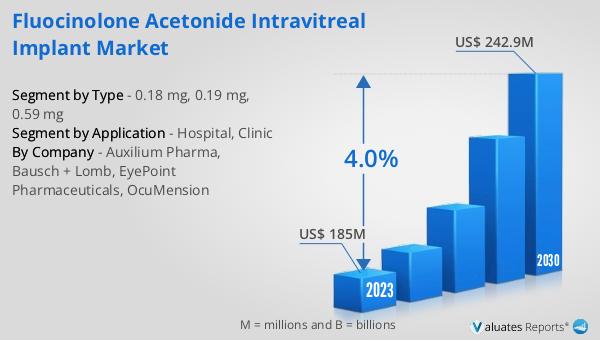
The global Fluocinolone Acetonide Intravitreal Implant market was valued at US$ 185 million in 2023 and is anticipated to reach US$ 242.9 million by 2030, witnessing a CAGR of 4.0% during the forecast period 2024-2030. The global pharmaceutical market was valued at 1475 billion USD in 2022, growing at a CAGR of 5% over the next six years. In comparison, the chemical drug market is estimated to increase from 1005 billion USD in 2018 to 1094 billion USD in 2022. This data highlights the significant growth potential of the Fluocinolone Acetonide Intravitreal Implant market within the broader pharmaceutical industry. The steady growth rate of the implant market reflects the increasing demand for effective treatments for severe eye conditions. The comparison with the chemical drug market also underscores the importance of specialized treatments like intravitreal implants in addressing specific medical needs. The growth of the pharmaceutical market as a whole indicates a robust demand for innovative and effective medical treatments, with the Fluocinolone Acetonide Intravitreal Implant market playing a crucial role in meeting the needs of patients with chronic and severe eye conditions. The market outlook for these implants is positive, driven by advancements in medical technology, increasing prevalence of eye diseases, and the growing aging population. The collaboration between healthcare providers, pharmaceutical companies, and regulatory bodies ensures the availability and accessibility of these essential treatments, contributing to the overall growth of the market.
| Report Metric | Details |
| Report Name | Fluocinolone Acetonide Intravitreal Implant Market |
| Accounted market size in 2023 | US$ 185 million |
| Forecasted market size in 2030 | US$ 242.9 million |
| CAGR | 4.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Auxilium Pharma, Bausch + Lomb, EyePoint Pharmaceuticals, OcuMension |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
