What is Global Chronic Inflammatory Demyelinating Polyneuropathy Drug Market?
The Global Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) Drug Market is a specialized segment within the broader pharmaceutical industry, focusing on the development and distribution of medications designed to treat CIDP. CIDP is a rare neurological disorder characterized by progressive weakness and impaired sensory function in the legs and arms. It is caused by damage to the myelin sheath, the protective covering of the nerves, due to chronic inflammation. The market for CIDP drugs is driven by the increasing prevalence of the disorder, advancements in medical research, and the growing awareness among healthcare professionals and patients about the condition. Pharmaceutical companies are investing in research and development to create effective treatments that can manage symptoms and improve the quality of life for patients. The market includes a variety of drugs, each with unique mechanisms of action, aimed at reducing inflammation, modulating the immune system, and promoting nerve repair. As the understanding of CIDP improves, the market is expected to evolve, offering new therapeutic options and improving patient outcomes.
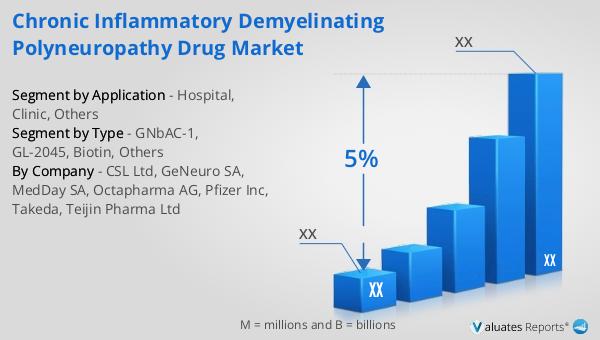
GNbAC-1, GL-2045, Biotin, Others in the Global Chronic Inflammatory Demyelinating Polyneuropathy Drug Market:
GNbAC-1, GL-2045, Biotin, and other drugs represent a diverse array of treatment options within the Global Chronic Inflammatory Demyelinating Polyneuropathy Drug Market. GNbAC-1 is a monoclonal antibody that targets specific proteins involved in the inflammatory process, aiming to reduce the immune system's attack on the myelin sheath. This drug is part of a new wave of biologics that offer targeted therapy, potentially leading to fewer side effects compared to traditional immunosuppressants. GL-2045 is another innovative treatment, designed as a recombinant protein that modulates the immune response. It works by mimicking certain natural proteins in the body, thereby reducing inflammation and preventing further nerve damage. This approach is particularly promising as it offers a novel mechanism of action that could complement existing therapies. Biotin, a form of vitamin B7, has gained attention for its potential role in nerve repair and myelin production. High doses of biotin are being investigated for their ability to enhance energy production in nerve cells, which may help in the remyelination process. This vitamin-based therapy is appealing due to its natural origin and the possibility of fewer adverse effects. Other drugs in the market include a range of immunoglobulins, corticosteroids, and plasmapheresis treatments. Immunoglobulins are used to modulate the immune system and are often administered intravenously. They are considered a first-line treatment for CIDP due to their effectiveness in reducing symptoms and improving nerve function. Corticosteroids, such as prednisone, are another common treatment option, working by suppressing the immune system to decrease inflammation. However, long-term use of corticosteroids can lead to significant side effects, prompting the need for alternative therapies. Plasmapheresis, or plasma exchange, is a procedure that removes antibodies from the blood, providing temporary relief from symptoms. While effective, it is typically reserved for severe cases or when other treatments have failed. The CIDP drug market is characterized by ongoing research and clinical trials aimed at discovering new therapies and improving existing ones. The development of combination therapies, which use multiple drugs to target different aspects of the disease, is an area of particular interest. As researchers continue to explore the underlying mechanisms of CIDP, the potential for new and more effective treatments grows, offering hope to patients and healthcare providers alike.
Hospital, Clinic, Others in the Global Chronic Inflammatory Demyelinating Polyneuropathy Drug Market:
The usage of drugs from the Global Chronic Inflammatory Demyelinating Polyneuropathy Drug Market is crucial in various healthcare settings, including hospitals, clinics, and other medical facilities. In hospitals, these drugs are often administered to patients with severe symptoms or those requiring intensive care. Hospitals provide the necessary infrastructure for administering intravenous treatments, such as immunoglobulins and plasmapheresis, which require specialized equipment and medical supervision. The hospital setting also allows for close monitoring of patients, ensuring that any adverse reactions to the drugs are promptly addressed. In clinics, CIDP drugs are used for ongoing management and follow-up care. Clinics offer a more accessible and less intensive environment for patients who require regular treatment but do not need hospitalization. Here, patients can receive corticosteroids or oral medications, which are easier to administer and manage on an outpatient basis. Clinics also play a vital role in patient education, helping individuals understand their condition and the importance of adhering to their treatment regimen. Other healthcare settings, such as rehabilitation centers and home care services, also utilize CIDP drugs to support patients in their recovery and daily living. Rehabilitation centers focus on helping patients regain strength and mobility, often incorporating drug therapy as part of a comprehensive treatment plan. Home care services provide an alternative for patients who prefer to receive treatment in the comfort of their own homes. This approach is particularly beneficial for those with mobility issues or who live far from medical facilities. Home care services can include the administration of oral medications, as well as coordination with healthcare providers for more complex treatments. The versatility of CIDP drugs allows them to be adapted to various healthcare settings, ensuring that patients receive the appropriate level of care based on their individual needs. As the understanding of CIDP and its treatment options continues to evolve, healthcare providers are better equipped to offer personalized care that improves patient outcomes and quality of life.
Global Chronic Inflammatory Demyelinating Polyneuropathy Drug Market Outlook:
The outlook for the Global Chronic Inflammatory Demyelinating Polyneuropathy Drug Market can be contextualized within the broader pharmaceutical industry trends. In 2022, the global pharmaceutical market was valued at approximately 1,475 billion USD, with an expected compound annual growth rate (CAGR) of 5% over the next six years. This growth is indicative of the increasing demand for innovative and effective treatments across various medical conditions, including CIDP. In comparison, the chemical drug market, a subset of the pharmaceutical industry, was projected to grow from 1,005 billion USD in 2018 to 1,094 billion USD by 2022. This growth reflects the ongoing advancements in drug development and the introduction of new therapies that address unmet medical needs. The CIDP drug market, as part of this larger landscape, benefits from these industry trends, as pharmaceutical companies continue to invest in research and development to discover new treatments and improve existing ones. The focus on biologics and targeted therapies, such as monoclonal antibodies and recombinant proteins, aligns with the broader shift towards personalized medicine and precision healthcare. As the pharmaceutical industry continues to evolve, the CIDP drug market is poised to offer more effective and tailored treatment options for patients, ultimately enhancing their quality of life.
| Report Metric | Details |
| Report Name | Chronic Inflammatory Demyelinating Polyneuropathy Drug Market |
| CAGR | 5% |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | CSL Ltd, GeNeuro SA, MedDay SA, Octapharma AG, Pfizer Inc, Takeda, Teijin Pharma Ltd |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |