What is Global cfDNA Testing Market?
The Global cfDNA Testing Market is a rapidly evolving sector within the healthcare industry, focusing on the analysis of cell-free DNA (cfDNA) present in the bloodstream. cfDNA refers to fragments of DNA that are released into the blood from cells undergoing apoptosis or necrosis. This market is gaining traction due to its non-invasive nature, which allows for the detection and monitoring of various health conditions without the need for invasive procedures like biopsies. The applications of cfDNA testing are vast, ranging from prenatal screening to cancer diagnostics and monitoring organ transplants. The technology behind cfDNA testing involves sophisticated techniques to isolate and analyze these DNA fragments, providing valuable insights into genetic information. As the demand for personalized medicine and early disease detection grows, the cfDNA testing market is expected to expand significantly, driven by advancements in genomic technologies and increasing awareness among healthcare providers and patients. The market's growth is also supported by ongoing research and development efforts aimed at improving the accuracy and reliability of cfDNA tests, making them a crucial tool in modern medical diagnostics.
Targeted Sequencing, Whole Genome Sequencing in the Global cfDNA Testing Market:
Targeted Sequencing and Whole Genome Sequencing are two pivotal methodologies within the Global cfDNA Testing Market, each offering unique advantages and applications. Targeted Sequencing focuses on analyzing specific regions of the genome, which are of particular interest due to their known associations with certain diseases or conditions. This approach is highly efficient and cost-effective, as it allows for the examination of relevant genetic markers without the need to sequence the entire genome. In the context of cfDNA testing, Targeted Sequencing is often employed in prenatal testing to identify chromosomal abnormalities such as Down syndrome, as well as in oncology to detect specific mutations associated with various cancers. By concentrating on predefined genomic regions, Targeted Sequencing provides precise and actionable insights, enabling healthcare providers to make informed decisions regarding patient care and treatment strategies. On the other hand, Whole Genome Sequencing (WGS) offers a comprehensive analysis of an individual's entire genetic makeup. This method involves sequencing all of the DNA in a sample, providing a complete picture of the genetic variations present. In the realm of cfDNA testing, WGS is particularly valuable for its ability to uncover rare or novel mutations that may not be detected through Targeted Sequencing. This makes it an indispensable tool in research settings, where a broader understanding of genetic factors is required. In oncology, WGS can reveal complex tumor profiles, aiding in the development of personalized treatment plans and the identification of potential therapeutic targets. Additionally, WGS is instrumental in prenatal testing, offering a detailed assessment of fetal genetic health and identifying potential risks that may not be apparent through more focused approaches. Despite its comprehensive nature, WGS is more resource-intensive and costly compared to Targeted Sequencing, which can limit its widespread adoption in routine clinical practice. However, ongoing advancements in sequencing technologies and cost reductions are gradually making WGS more accessible, expanding its potential applications in the cfDNA testing market. Both Targeted Sequencing and Whole Genome Sequencing play crucial roles in advancing the capabilities of cfDNA testing, each contributing to the broader goal of enhancing diagnostic accuracy and improving patient outcomes. As the field continues to evolve, the integration of these methodologies with emerging technologies such as artificial intelligence and machine learning is expected to further enhance their utility, paving the way for more precise and personalized healthcare solutions.
Prenatal Testing, Oncology, Organ Transplant Monitoring, Others in the Global cfDNA Testing Market:
The Global cfDNA Testing Market finds its applications across several critical areas, including Prenatal Testing, Oncology, Organ Transplant Monitoring, and others, each benefiting from the non-invasive and highly informative nature of cfDNA analysis. In Prenatal Testing, cfDNA testing is revolutionizing the way expectant parents and healthcare providers approach prenatal care. By analyzing cfDNA from the maternal bloodstream, it is possible to screen for chromosomal abnormalities such as Down syndrome, Edwards syndrome, and Patau syndrome with high accuracy and minimal risk to the fetus. This non-invasive prenatal testing (NIPT) offers a safer alternative to traditional invasive procedures like amniocentesis, reducing the risk of miscarriage and providing peace of mind to parents. In the field of Oncology, cfDNA testing is emerging as a powerful tool for cancer detection, monitoring, and treatment planning. Tumor-derived cfDNA, often referred to as circulating tumor DNA (ctDNA), can be isolated from a patient's blood sample, providing insights into the genetic mutations driving cancer progression. This allows for the early detection of cancer, monitoring of treatment response, and identification of potential resistance mechanisms, enabling oncologists to tailor therapies to individual patients and improve outcomes. Organ Transplant Monitoring is another area where cfDNA testing is making significant strides. After a transplant, the presence of donor-derived cfDNA in the recipient's bloodstream can serve as an indicator of organ rejection. By regularly monitoring cfDNA levels, healthcare providers can detect signs of rejection early, allowing for timely intervention and potentially improving transplant success rates. Beyond these specific applications, cfDNA testing is also being explored in other areas such as infectious disease detection, where it can aid in identifying pathogens and monitoring treatment efficacy. The versatility and non-invasive nature of cfDNA testing make it a valuable tool across a wide range of medical disciplines, contributing to the advancement of personalized medicine and improving patient care. As research continues to uncover new applications and refine existing methodologies, the Global cfDNA Testing Market is poised to play an increasingly important role in modern healthcare.
Global cfDNA Testing Market Outlook:
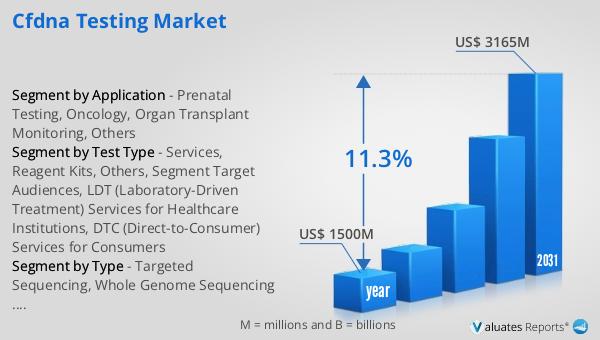
The global cfDNA Testing Market is on a promising trajectory, with its valuation standing at US$ 1500 million in 2024. This market is anticipated to experience substantial growth, reaching an estimated size of US$ 3165 million by 2031. This expansion is driven by a compound annual growth rate (CAGR) of 11.3% during the forecast period. The robust growth of the cfDNA Testing Market can be attributed to several factors, including the increasing demand for non-invasive diagnostic tools, advancements in genomic technologies, and a growing emphasis on personalized medicine. As healthcare providers and patients alike seek more accurate and less invasive methods for disease detection and monitoring, cfDNA testing offers a compelling solution. The market's expansion is further supported by ongoing research and development efforts aimed at enhancing the accuracy, reliability, and accessibility of cfDNA tests. These efforts are crucial in addressing the diverse needs of various medical fields, from prenatal screening to oncology and organ transplant monitoring. As the cfDNA Testing Market continues to evolve, it is expected to play a pivotal role in shaping the future of medical diagnostics, offering new possibilities for early disease detection, personalized treatment plans, and improved patient outcomes. The projected growth of this market underscores the increasing recognition of cfDNA testing as a valuable tool in modern healthcare, paving the way for more precise and personalized medical solutions.
| Report Metric | Details |
| Report Name | cfDNA Testing Market |
| Accounted market size in year | US$ 1500 million |
| Forecasted market size in 2031 | US$ 3165 million |
| CAGR | 11.3% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Test Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Illumina, Oncogenomics, Revvity, CareDx, Natera, BillionToOne, Inc., Yourgene Health, ARUP Laboratories, Roche |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
