What is Global Medical PTFE Coated Guidewire Market?
The Global Medical PTFE Coated Guidewire Market is a specialized segment within the broader medical device industry, focusing on guidewires that are coated with polytetrafluoroethylene (PTFE). These guidewires are essential tools in various medical procedures, particularly in minimally invasive surgeries and diagnostic procedures. PTFE is a high-performance fluoropolymer known for its non-stick properties, chemical resistance, and low friction, making it an ideal coating for guidewires used in delicate medical procedures. The market for these guidewires is driven by the increasing demand for minimally invasive surgeries, advancements in medical technology, and the growing prevalence of chronic diseases that require surgical intervention. As healthcare systems worldwide continue to evolve, the need for efficient, reliable, and safe medical devices like PTFE coated guidewires is expected to rise. These guidewires are used in a variety of applications, including cardiovascular, neurovascular, and peripheral vascular procedures, highlighting their versatility and importance in modern medicine. The market is characterized by continuous innovation, with manufacturers focusing on improving the performance and safety of these devices to meet the stringent regulatory standards and the ever-growing expectations of healthcare professionals.
Fixed Core Guidewire, Moveable Core Guidewire in the Global Medical PTFE Coated Guidewire Market:
In the realm of the Global Medical PTFE Coated Guidewire Market, two primary types of guidewires are prevalent: Fixed Core Guidewires and Moveable Core Guidewires. Fixed Core Guidewires are designed with a solid core that provides stability and support during medical procedures. This type of guidewire is particularly useful in situations where rigidity and strength are required to navigate through complex anatomical pathways. The fixed core design ensures that the guidewire maintains its shape and position, which is crucial in procedures such as angioplasty or stent placement where precision is paramount. On the other hand, Moveable Core Guidewires offer greater flexibility and maneuverability. These guidewires have a core that can be adjusted or moved, allowing for better navigation through tortuous vessels or challenging anatomical structures. The moveable core design is advantageous in procedures where adaptability and responsiveness are needed, such as in neurovascular interventions or when accessing difficult-to-reach areas within the body. Both types of guidewires are coated with PTFE to enhance their performance by reducing friction and improving their ability to glide smoothly through blood vessels. This coating also minimizes the risk of damage to the vessel walls, thereby enhancing patient safety. The choice between a fixed core and a moveable core guidewire depends on the specific requirements of the procedure and the preferences of the healthcare professional. In the Global Medical PTFE Coated Guidewire Market, manufacturers are continually innovating to improve the design and functionality of both fixed and moveable core guidewires. This includes the development of guidewires with enhanced torque control, improved visibility under imaging, and increased biocompatibility. These advancements aim to provide healthcare professionals with the tools they need to perform procedures with greater accuracy and efficiency. Additionally, the market is witnessing a trend towards the customization of guidewires to meet the specific needs of different medical specialties and procedures. This customization can involve variations in the diameter, length, and flexibility of the guidewires, as well as the incorporation of additional features such as hydrophilic coatings or radiopaque markers. As the demand for minimally invasive procedures continues to grow, the Global Medical PTFE Coated Guidewire Market is expected to expand, driven by the need for high-quality, reliable, and versatile guidewires that can meet the diverse needs of modern healthcare.
Operating Room, Medical Laboratory, Others in the Global Medical PTFE Coated Guidewire Market:
The usage of Global Medical PTFE Coated Guidewire Market products spans several critical areas in healthcare, including the Operating Room, Medical Laboratory, and other specialized settings. In the Operating Room, PTFE coated guidewires are indispensable tools for surgeons performing minimally invasive procedures. These guidewires facilitate the precise navigation of surgical instruments through the body's complex vascular system, enabling surgeons to reach target areas with minimal disruption to surrounding tissues. The low-friction PTFE coating ensures that the guidewires can glide smoothly through blood vessels, reducing the risk of injury and improving the overall safety and efficacy of the procedure. In cardiovascular surgeries, for example, PTFE coated guidewires are used to guide catheters and stents to the site of a blockage, allowing for the restoration of blood flow with minimal trauma to the vessel walls. In the Medical Laboratory, PTFE coated guidewires play a crucial role in diagnostic procedures. These guidewires are used in conjunction with imaging technologies such as angiography or fluoroscopy to visualize and assess the condition of blood vessels and other anatomical structures. The PTFE coating enhances the visibility of the guidewires under imaging, allowing for more accurate diagnosis and treatment planning. In addition to their use in the Operating Room and Medical Laboratory, PTFE coated guidewires are also employed in a variety of other medical settings. For instance, they are used in interventional radiology to facilitate the delivery of therapeutic agents directly to the site of a disease, such as in the treatment of tumors or vascular malformations. They are also used in endoscopic procedures to navigate through the gastrointestinal tract or other hollow organs. The versatility and reliability of PTFE coated guidewires make them an essential component of many medical procedures, contributing to improved patient outcomes and reduced recovery times. As the healthcare industry continues to advance, the demand for high-quality PTFE coated guidewires is expected to grow, driven by the increasing prevalence of chronic diseases, the aging population, and the ongoing shift towards minimally invasive surgical techniques.
Global Medical PTFE Coated Guidewire Market Outlook:
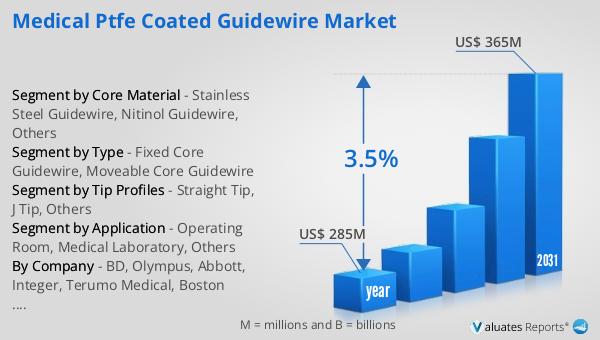
The global market for Medical PTFE Coated Guidewire was valued at $285 million in 2024, and it is anticipated to grow to a revised size of $365 million by 2031, reflecting a compound annual growth rate (CAGR) of 3.5% during the forecast period. This growth trajectory underscores the increasing demand for PTFE coated guidewires in the medical field, driven by advancements in medical technology and the rising prevalence of conditions that require surgical intervention. The steady growth rate indicates a robust market with opportunities for innovation and expansion. As healthcare systems worldwide continue to evolve, the need for efficient, reliable, and safe medical devices like PTFE coated guidewires is expected to rise. The market's expansion is also fueled by the growing adoption of minimally invasive surgical techniques, which rely heavily on the use of guidewires for precise navigation and placement of medical instruments. Furthermore, the increasing focus on improving patient outcomes and reducing recovery times is driving the demand for high-quality guidewires that can enhance the safety and efficacy of medical procedures. As a result, manufacturers are investing in research and development to create advanced guidewires with improved performance characteristics, such as enhanced flexibility, torque control, and biocompatibility. The global market for Medical PTFE Coated Guidewire is poised for continued growth, offering significant opportunities for stakeholders in the healthcare industry.
| Report Metric | Details |
| Report Name | Medical PTFE Coated Guidewire Market |
| Accounted market size in year | US$ 285 million |
| Forecasted market size in 2031 | US$ 365 million |
| CAGR | 3.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Core Material |
|
| Segment by Tip Profiles |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | BD, Olympus, Abbott, Integer, Terumo Medical, Boston Scientific, Medtronic, Merit Medical, SCW Medicath, Coloplast, Seplou Medical, Argon Medical Devices, Surface Solutions Group (SSG), Advin Healthcare, SP Medical, Tianck Medical, EPflex, Wytech, Teleflex, KT Medical, SCITECH, Dispack Medical, Acme Monaco, Newtech Medical Devices, Shunmei Medical, MicroApproach Medical |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
