What is Global Mesalazine API Market?
The Global Mesalazine API Market refers to the worldwide industry focused on the production and distribution of Mesalazine, an active pharmaceutical ingredient (API) primarily used in the treatment of inflammatory bowel diseases such as ulcerative colitis and Crohn's disease. Mesalazine, also known as 5-aminosalicylic acid (5-ASA), works by reducing inflammation in the colon, thereby alleviating symptoms associated with these chronic conditions. The market encompasses various stages of the supply chain, including raw material procurement, manufacturing, and distribution to pharmaceutical companies that formulate the final medicinal products. The demand for Mesalazine API is driven by the increasing prevalence of gastrointestinal disorders globally, advancements in drug formulation technologies, and the growing awareness of effective treatment options. Additionally, the market is influenced by regulatory standards and the need for high-quality production processes to ensure the safety and efficacy of the API. As healthcare systems worldwide continue to prioritize the management of chronic diseases, the Global Mesalazine API Market is expected to experience steady growth, with key players investing in research and development to enhance product offerings and expand their market presence.
Above 97 %, Above 98 %, Above 99 % in the Global Mesalazine API Market:
In the Global Mesalazine API Market, the purity levels of the API are critical, with categories typically defined as Above 97%, Above 98%, and Above 99%. These purity levels indicate the concentration of Mesalazine in the API, which directly impacts the efficacy and safety of the final pharmaceutical products. APIs with a purity level of Above 97% are generally considered suitable for most therapeutic applications, providing a balance between cost-effectiveness and therapeutic efficacy. This level of purity is often used in generic formulations where cost constraints are a significant consideration. However, as the demand for higher efficacy and safety standards increases, there is a growing preference for APIs with higher purity levels. APIs with a purity of Above 98% offer enhanced therapeutic benefits, as the higher concentration of the active ingredient can lead to improved patient outcomes. This level of purity is often preferred in branded formulations and in markets where regulatory standards are stringent. The highest purity level, Above 99%, represents the pinnacle of quality in the Mesalazine API Market. APIs at this level are used in premium pharmaceutical products, where the utmost precision in dosage and minimal side effects are paramount. These high-purity APIs are often employed in innovative drug formulations and in regions with the most rigorous regulatory requirements. The production of high-purity Mesalazine APIs involves advanced manufacturing processes and stringent quality control measures to ensure consistency and reliability. As the pharmaceutical industry continues to evolve, the demand for high-purity APIs is expected to rise, driven by the need for more effective and safer treatment options. Manufacturers in the Global Mesalazine API Market are investing in state-of-the-art technologies and research to achieve these high purity levels, thereby enhancing their competitive edge and meeting the growing expectations of healthcare providers and patients alike. The choice of purity level in Mesalazine APIs is influenced by various factors, including regulatory requirements, therapeutic goals, and market dynamics. In regions with strict regulatory frameworks, such as Europe and North America, higher purity levels are often mandated to ensure patient safety and drug efficacy. In contrast, emerging markets may prioritize cost-effectiveness, leading to a preference for APIs with slightly lower purity levels. However, as global standards continue to harmonize, there is a noticeable shift towards higher purity APIs across all regions. This trend is further supported by the increasing prevalence of inflammatory bowel diseases and the need for more effective treatment options. The Global Mesalazine API Market is thus characterized by a dynamic interplay between purity levels, regulatory standards, and market demands, with manufacturers striving to balance these factors to deliver high-quality APIs that meet the diverse needs of the pharmaceutical industry.
Tablets, Capsules, Granule, Suppository, Enema, Others in the Global Mesalazine API Market:
The Global Mesalazine API Market finds its application in various pharmaceutical forms, including tablets, capsules, granules, suppositories, enemas, and others. Each form offers unique advantages and is chosen based on the specific needs of patients and the nature of the condition being treated. Tablets are one of the most common forms of Mesalazine medication, offering convenience and ease of administration. They are typically used for the long-term management of inflammatory bowel diseases, providing a controlled release of the active ingredient to maintain therapeutic levels in the colon. Capsules, similar to tablets, offer a convenient oral dosage form but may provide different release profiles, such as delayed or extended release, to optimize the delivery of Mesalazine to the affected areas of the gastrointestinal tract. Granules are another oral form, often used for patients who have difficulty swallowing tablets or capsules. They can be mixed with food or liquids, providing flexibility in administration and ensuring patient compliance. Suppositories are used for localized treatment, delivering Mesalazine directly to the rectal area. This form is particularly beneficial for patients with proctitis or distal ulcerative colitis, where the inflammation is confined to the lower part of the colon. Enemas, on the other hand, are liquid formulations administered rectally to treat inflammation in the distal colon and rectum. They provide a higher concentration of the active ingredient directly to the affected area, offering rapid relief from symptoms. Other forms of Mesalazine medication may include topical formulations or combination therapies, designed to address specific patient needs or enhance therapeutic outcomes. The choice of formulation in the Global Mesalazine API Market is influenced by various factors, including the severity and location of the disease, patient preferences, and physician recommendations. Manufacturers are continually innovating to develop new formulations that improve patient adherence, enhance drug delivery, and minimize side effects. As the understanding of inflammatory bowel diseases evolves, the demand for diverse and effective Mesalazine formulations is expected to grow, driving further advancements in the Global Mesalazine API Market.
Global Mesalazine API Market Outlook:
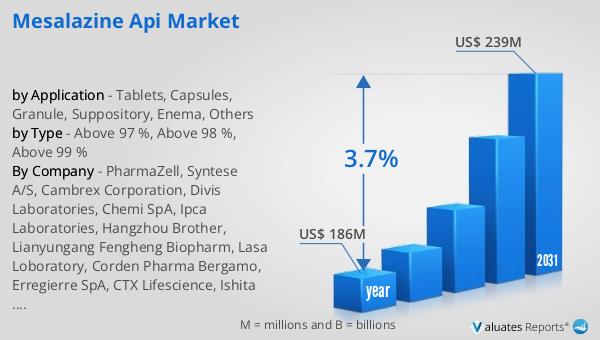
The global market for Mesalazine API was valued at $186 million in 2024 and is anticipated to expand to a revised size of $239 million by 2031, reflecting a compound annual growth rate (CAGR) of 3.7% over the forecast period. The market is dominated by the top three companies, which collectively hold a share exceeding 50%. Europe emerges as the largest market, accounting for approximately 43% of the global share, followed by the Asia Pacific region and North America, which hold shares of 31% and 12%, respectively. This distribution highlights the significant demand for Mesalazine API in Europe, driven by the region's advanced healthcare infrastructure and high prevalence of inflammatory bowel diseases. The Asia Pacific region, with its rapidly growing pharmaceutical industry and increasing awareness of gastrointestinal health, presents substantial growth opportunities for market players. North America's share, while smaller, is supported by the region's strong focus on healthcare innovation and the presence of leading pharmaceutical companies. The competitive landscape of the Global Mesalazine API Market is characterized by strategic collaborations, mergers, and acquisitions among key players, aimed at expanding their market presence and enhancing product offerings. As the market continues to evolve, companies are investing in research and development to improve the quality and efficacy of Mesalazine APIs, catering to the diverse needs of healthcare providers and patients worldwide. The steady growth of the Global Mesalazine API Market underscores the increasing importance of effective treatment options for inflammatory bowel diseases and the ongoing efforts of industry players to meet this demand through innovation and strategic market positioning.
| Report Metric | Details |
| Report Name | Mesalazine API Market |
| Accounted market size in year | US$ 186 million |
| Forecasted market size in 2031 | US$ 239 million |
| CAGR | 3.7% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | PharmaZell, Syntese A/S, Cambrex Corporation, Divis Laboratories, Chemi SpA, Ipca Laboratories, Hangzhou Brother, Lianyungang Fengheng Biopharm, Lasa Loboratory, Corden Pharma Bergamo, Erregierre SpA, CTX Lifescience, Ishita Active Pharma Ingredients, YC Biotech (Jiangsu), Xinxiang Tianfeng Fine Chemical |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |