What is Global Multi-Spike Viral Clearance Market?
The Global Multi-Spike Viral Clearance Market is a specialized sector within the broader pharmaceutical and biotechnology industries, focusing on the removal or inactivation of viral contaminants from biological products. This market is crucial for ensuring the safety and efficacy of biopharmaceuticals, vaccines, and other biologically derived products. Viral clearance is a mandatory step in the production process, as it ensures that the final product is free from any viral particles that could pose a risk to human health. The market encompasses a range of technologies and methods designed to detect, remove, or inactivate viruses, thereby safeguarding the integrity of the product. These methods are essential for compliance with stringent regulatory standards set by health authorities worldwide. The market is driven by the increasing demand for biopharmaceuticals and vaccines, advancements in biotechnology, and the growing emphasis on product safety. As the biopharmaceutical industry continues to expand, the need for effective viral clearance solutions becomes even more critical. This market is characterized by continuous innovation, with companies investing in research and development to enhance the efficiency and reliability of viral clearance technologies. Overall, the Global Multi-Spike Viral Clearance Market plays a vital role in the pharmaceutical supply chain, ensuring that products are safe for human use.
Physical Removal Techniques, Chemical Inactivation Techniques, Others in the Global Multi-Spike Viral Clearance Market:
The Global Multi-Spike Viral Clearance Market employs various techniques to ensure the removal or inactivation of viral contaminants from biological products. These techniques are broadly categorized into physical removal techniques, chemical inactivation techniques, and others. Physical removal techniques primarily involve filtration and chromatography. Filtration is a widely used method where viral particles are physically separated from the product using filters with specific pore sizes. This method is effective for removing larger viruses and is often used in conjunction with other techniques to ensure comprehensive viral clearance. Chromatography, on the other hand, involves the separation of viruses based on their size, charge, or other properties. This technique is highly effective for purifying complex biological mixtures and is commonly used in the production of biopharmaceuticals. Chemical inactivation techniques involve the use of chemical agents to inactivate viruses. These agents disrupt the viral structure, rendering the virus non-infectious. Common chemical inactivation methods include the use of detergents, solvents, and pH adjustments. Detergents, such as Triton X-100, are used to solubilize viral envelopes, effectively inactivating enveloped viruses. Solvent/detergent treatment is another widely used method, particularly for plasma-derived products, where a combination of solvents and detergents is used to inactivate lipid-enveloped viruses. pH adjustments involve altering the pH of the product to levels that are unfavorable for viral survival, thereby inactivating the virus. Other techniques in the Global Multi-Spike Viral Clearance Market include heat treatment and ultraviolet (UV) irradiation. Heat treatment involves exposing the product to high temperatures for a specific period, effectively inactivating heat-sensitive viruses. This method is commonly used for products that can withstand high temperatures without degradation. UV irradiation involves exposing the product to UV light, which damages the viral DNA or RNA, preventing replication. This method is effective for inactivating a wide range of viruses and is often used in combination with other techniques to enhance viral clearance. Each of these techniques has its advantages and limitations, and the choice of method depends on the specific requirements of the product and the type of viruses that need to be cleared. The Global Multi-Spike Viral Clearance Market is characterized by continuous innovation, with companies investing in research and development to enhance the efficiency and reliability of these techniques. As the demand for biopharmaceuticals and vaccines continues to grow, the need for effective viral clearance solutions becomes even more critical. Overall, the market plays a vital role in ensuring the safety and efficacy of biological products, safeguarding public health.
Biopharmaceuticals, Vaccine Production, Research Institutes, Others in the Global Multi-Spike Viral Clearance Market:
The Global Multi-Spike Viral Clearance Market finds extensive application in various areas, including biopharmaceuticals, vaccine production, research institutes, and others. In the biopharmaceutical sector, viral clearance is a critical step in the production process. Biopharmaceuticals, which include monoclonal antibodies, recombinant proteins, and cell and gene therapies, are derived from living organisms and are therefore susceptible to viral contamination. Ensuring viral clearance is essential to guarantee the safety and efficacy of these products. The market provides a range of technologies and methods to detect, remove, or inactivate viruses, thereby ensuring that the final product is free from any viral particles that could pose a risk to human health. In vaccine production, viral clearance is equally important. Vaccines are biological preparations that provide immunity against infectious diseases, and their production involves the use of live or attenuated viruses. The Global Multi-Spike Viral Clearance Market offers solutions to ensure that any viral contaminants are effectively removed or inactivated, safeguarding the integrity of the vaccine. This is crucial for maintaining public trust in vaccination programs and ensuring that vaccines are safe for human use. Research institutes also rely on viral clearance technologies for their work. These institutes conduct research on various biological products, including new drugs, vaccines, and therapies. Ensuring that their research materials are free from viral contaminants is essential for the accuracy and reliability of their findings. The Global Multi-Spike Viral Clearance Market provides the necessary tools and technologies to support this research, enabling scientists to conduct their work with confidence. Other areas where the market finds application include blood and plasma products, tissue and organ transplants, and diagnostic products. In each of these areas, ensuring viral clearance is essential to protect human health and ensure the safety and efficacy of the products. The market offers a range of solutions to meet the specific needs of these applications, ensuring that viral contaminants are effectively removed or inactivated. Overall, the Global Multi-Spike Viral Clearance Market plays a vital role in various sectors, ensuring that biological products are safe for human use and supporting the development of new therapies and vaccines.
Global Multi-Spike Viral Clearance Market Outlook:
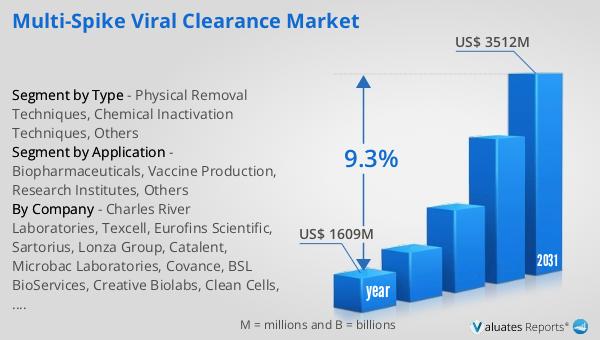
The global market for Multi-Spike Viral Clearance was valued at $1,609 million in 2024 and is anticipated to grow significantly, reaching an estimated size of $3,512 million by 2031. This growth is expected to occur at a compound annual growth rate (CAGR) of 9.3% over the forecast period. This impressive growth trajectory underscores the increasing importance of viral clearance technologies in the pharmaceutical and biotechnology industries. As the demand for biopharmaceuticals and vaccines continues to rise, driven by advancements in biotechnology and the growing emphasis on product safety, the need for effective viral clearance solutions becomes even more critical. The market's expansion is also fueled by continuous innovation, with companies investing in research and development to enhance the efficiency and reliability of viral clearance technologies. This investment is crucial for meeting the stringent regulatory standards set by health authorities worldwide and ensuring that biological products are safe for human use. The projected growth of the Global Multi-Spike Viral Clearance Market reflects the vital role it plays in the pharmaceutical supply chain, safeguarding public health and supporting the development of new therapies and vaccines. As the market continues to evolve, it will remain a key component of the biopharmaceutical industry, ensuring that products are free from viral contaminants and safe for human use.
| Report Metric | Details |
| Report Name | Multi-Spike Viral Clearance Market |
| Accounted market size in year | US$ 1609 million |
| Forecasted market size in 2031 | US$ 3512 million |
| CAGR | 9.3% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Charles River Laboratories, Texcell, Eurofins Scientific, Sartorius, Lonza Group, Catalent, Microbac Laboratories, Covance, BSL BioServices, Creative Biolabs, Clean Cells, ViruSure GmbH, Vironova |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
