What is Global Gene Editing Drug Market?
The Global Gene Editing Drug Market refers to the worldwide industry focused on developing and commercializing drugs that utilize gene editing technologies to treat various genetic disorders and diseases. These drugs are designed to precisely alter the DNA within a patient's cells, thereby correcting genetic mutations or introducing new genetic material to combat disease. The market encompasses a range of gene editing techniques, including CRISPR-Cas9, Zinc Finger Nucleases (ZFNs), and other advanced methods. These technologies have the potential to revolutionize the treatment of genetic conditions by offering more targeted and effective therapies compared to traditional treatments. The market is driven by increasing investments in biotechnology research, growing prevalence of genetic disorders, and advancements in gene editing technologies. As a result, the Global Gene Editing Drug Market is poised for significant growth, with numerous pharmaceutical companies and research institutions actively developing new gene editing therapies.
CRISPR-Cas9 Gene Editing Drugs, Zinc Finger Nuclease Gene Editing Drugs, Others in the Global Gene Editing Drug Market:
CRISPR-Cas9 Gene Editing Drugs represent a significant advancement in the field of gene therapy. CRISPR-Cas9 is a revolutionary technology that allows scientists to make precise, targeted changes to the DNA of living organisms. This method involves using a specialized protein (Cas9) and a guide RNA to locate and cut specific DNA sequences, enabling the addition, removal, or alteration of genetic material. CRISPR-Cas9 has shown immense potential in treating a variety of genetic disorders, including cystic fibrosis, sickle cell anemia, and certain types of cancer. The simplicity, efficiency, and versatility of CRISPR-Cas9 make it a popular choice among researchers and pharmaceutical companies. Zinc Finger Nuclease (ZFN) Gene Editing Drugs, on the other hand, utilize engineered proteins called zinc finger nucleases to target and modify specific DNA sequences. ZFNs work by binding to DNA and creating double-strand breaks, which can then be repaired by the cell's natural repair mechanisms, leading to the desired genetic modification. ZFNs have been used in clinical trials for conditions such as HIV, hemophilia, and beta-thalassemia. Although ZFNs are less versatile than CRISPR-Cas9, they offer a high degree of specificity and have been successfully used in various therapeutic applications. Other gene editing technologies, such as TALENs (Transcription Activator-Like Effector Nucleases) and meganucleases, also play a role in the Global Gene Editing Drug Market. TALENs function similarly to ZFNs but use different proteins to recognize and bind to DNA sequences. Meganucleases, derived from microbial enzymes, are highly specific DNA-cutting enzymes that can be engineered to target specific genetic sequences. Each of these technologies has its own set of advantages and limitations, and ongoing research aims to optimize their use for therapeutic purposes. The development of gene editing drugs involves rigorous testing and validation to ensure safety and efficacy. Preclinical studies in cell cultures and animal models are followed by clinical trials in humans to assess the therapeutic potential and identify any adverse effects. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), play a crucial role in overseeing the approval process for gene editing drugs. The approval and commercialization of these drugs require extensive collaboration between researchers, pharmaceutical companies, and regulatory bodies. The Global Gene Editing Drug Market is characterized by a high level of innovation and competition, with numerous companies striving to develop breakthrough therapies. Strategic partnerships, mergers, and acquisitions are common as companies seek to leverage each other's expertise and resources. Additionally, ethical considerations and public perception play a significant role in the development and acceptance of gene editing drugs. Ensuring that these therapies are used responsibly and equitably is essential for gaining public trust and support. In conclusion, CRISPR-Cas9, Zinc Finger Nucleases, and other gene editing technologies are at the forefront of the Global Gene Editing Drug Market. These innovative approaches hold great promise for treating a wide range of genetic disorders and improving patient outcomes. As research and development efforts continue, the market is expected to grow, offering new hope for patients with previously untreatable conditions.
General Hospital, Specialty Hospital in the Global Gene Editing Drug Market:
The usage of gene editing drugs in general hospitals and specialty hospitals is becoming increasingly prevalent as these institutions seek to provide cutting-edge treatments for their patients. General hospitals, which offer a wide range of medical services to the public, are beginning to incorporate gene editing therapies into their treatment protocols. These hospitals often serve as the first point of contact for patients with genetic disorders, making them an ideal setting for the initial diagnosis and referral to specialized care. In general hospitals, gene editing drugs can be used to treat a variety of conditions, including inherited genetic disorders, cancers, and infectious diseases. For example, patients with sickle cell anemia or cystic fibrosis may benefit from gene editing therapies that correct the underlying genetic mutations responsible for these conditions. Additionally, gene editing drugs can be used to enhance the effectiveness of existing treatments, such as chemotherapy or antiviral therapies, by targeting specific genetic pathways involved in disease progression. The integration of gene editing drugs into general hospital settings requires significant investment in infrastructure, training, and regulatory compliance. Healthcare providers must be equipped with the necessary knowledge and skills to administer these therapies safely and effectively. This includes understanding the mechanisms of action, potential side effects, and appropriate patient selection criteria. Furthermore, general hospitals must establish protocols for monitoring and managing patients undergoing gene editing treatments to ensure optimal outcomes. Specialty hospitals, which focus on specific areas of medicine such as oncology, neurology, or genetics, are also playing a crucial role in the adoption of gene editing drugs. These institutions often have access to advanced technologies and specialized expertise, making them well-suited for the development and implementation of gene editing therapies. In specialty hospitals, gene editing drugs are being used to address complex and rare genetic disorders that may not be adequately treated with conventional therapies. For instance, oncology centers are exploring the use of gene editing drugs to target specific genetic mutations driving cancer growth and resistance to treatment. By precisely altering the genetic makeup of cancer cells, these therapies have the potential to improve treatment outcomes and reduce the risk of relapse. Similarly, neurology centers are investigating gene editing approaches to treat neurodegenerative diseases such as Huntington's disease and amyotrophic lateral sclerosis (ALS). The use of gene editing drugs in specialty hospitals also involves collaboration with research institutions and pharmaceutical companies to conduct clinical trials and advance the development of new therapies. These partnerships are essential for translating scientific discoveries into clinical practice and ensuring that patients have access to the latest treatment options. Additionally, specialty hospitals often serve as centers of excellence for gene editing therapies, providing training and education to healthcare professionals and contributing to the broader dissemination of knowledge and best practices. In conclusion, the integration of gene editing drugs into general and specialty hospitals is transforming the landscape of medical treatment. These institutions are at the forefront of adopting innovative therapies that have the potential to revolutionize the management of genetic disorders and improve patient outcomes. As the Global Gene Editing Drug Market continues to evolve, the role of hospitals in delivering these cutting-edge treatments will become increasingly important.
Global Gene Editing Drug Market Outlook:
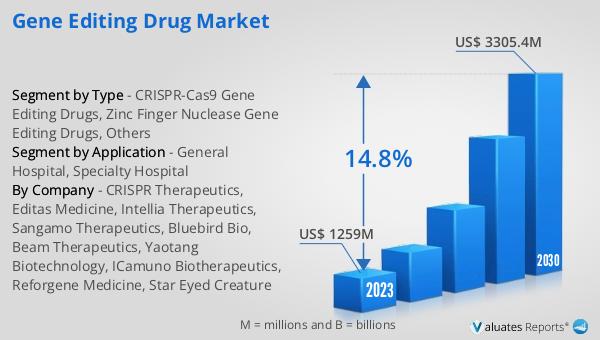
The global Gene Editing Drug market was valued at US$ 1259 million in 2023 and is anticipated to reach US$ 3305.4 million by 2030, witnessing a CAGR of 14.8% during the forecast period 2024-2030. This significant growth reflects the increasing demand for innovative therapies that can address a wide range of genetic disorders and diseases. The market's expansion is driven by advancements in gene editing technologies, growing investments in biotechnology research, and the rising prevalence of genetic conditions. As pharmaceutical companies and research institutions continue to develop and commercialize new gene editing drugs, the market is expected to experience substantial growth. The projected increase in market value underscores the potential of gene editing therapies to revolutionize the treatment of genetic disorders and improve patient outcomes. With a compound annual growth rate (CAGR) of 14.8%, the Global Gene Editing Drug Market is poised for significant advancements and breakthroughs in the coming years.
| Report Metric | Details |
| Report Name | Gene Editing Drug Market |
| Accounted market size in 2023 | US$ 1259 million |
| Forecasted market size in 2030 | US$ 3305.4 million |
| CAGR | 14.8% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | CRISPR Therapeutics, Editas Medicine, Intellia Therapeutics, Sangamo Therapeutics, Bluebird Bio, Beam Therapeutics, Yaotang Biotechnology, ICamuno Biotherapeutics, Reforgene Medicine, Star Eyed Creature |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
