What is Global Biopharmaceutical CDMO Service Market?
The Global Biopharmaceutical CDMO (Contract Development and Manufacturing Organization) Service Market is a specialized sector within the pharmaceutical industry that focuses on providing comprehensive services for the development and manufacturing of biopharmaceutical products. These services include everything from early-stage drug development to large-scale commercial manufacturing. CDMOs offer expertise in various areas such as cell line development, process development, analytical testing, and regulatory support. By outsourcing these critical functions to CDMOs, biopharmaceutical companies can focus on their core competencies, such as research and development, while leveraging the specialized skills and infrastructure of CDMOs to bring their products to market more efficiently. This market is driven by the increasing demand for biopharmaceuticals, which are complex and require specialized manufacturing processes. The global reach of CDMOs allows for the production and distribution of biopharmaceuticals on a scale that meets the needs of a growing global population. As the biopharmaceutical industry continues to expand, the role of CDMOs becomes increasingly vital in ensuring the timely and cost-effective delivery of innovative therapies to patients worldwide.
Develop Innovative Drugs, Listed Patented Drug, Biosimilars, Others in the Global Biopharmaceutical CDMO Service Market:
Developing innovative drugs is a cornerstone of the Global Biopharmaceutical CDMO Service Market. These drugs often target unmet medical needs and require advanced technologies and expertise to bring them from concept to reality. CDMOs play a crucial role in this process by providing the necessary infrastructure and technical know-how to develop these complex molecules. Listed patented drugs are another significant segment within this market. These are drugs that have been granted patent protection, giving the original developers exclusive rights to manufacture and sell them for a certain period. CDMOs assist in the production of these drugs, ensuring that they meet stringent regulatory standards and are produced at a scale that meets market demand. Biosimilars, which are essentially generic versions of biologic drugs, represent a growing area within the biopharmaceutical industry. Developing biosimilars requires a deep understanding of the original biologic drug's structure and function, as well as the ability to replicate its effects in a cost-effective manner. CDMOs provide the expertise and facilities needed to develop and manufacture biosimilars, helping to bring more affordable treatment options to market. Other services offered by CDMOs include the development and manufacturing of personalized medicines, vaccines, and gene therapies. Personalized medicines are tailored to individual patients based on their genetic makeup, requiring highly specialized manufacturing processes. Vaccines, which are critical for preventing infectious diseases, also require sophisticated production techniques to ensure their safety and efficacy. Gene therapies, which involve modifying a patient's genetic material to treat or cure diseases, represent one of the most cutting-edge areas of biopharmaceutical research. CDMOs provide the advanced technologies and expertise needed to develop and manufacture these innovative therapies. Overall, the Global Biopharmaceutical CDMO Service Market is essential for the development and production of a wide range of biopharmaceutical products, from innovative drugs and patented medicines to biosimilars and personalized therapies. By offering specialized services and expertise, CDMOs enable biopharmaceutical companies to bring new treatments to market more efficiently and cost-effectively, ultimately benefiting patients worldwide.
Pharmaceutical Company, Biotechnology Company, Others in the Global Biopharmaceutical CDMO Service Market:
The usage of Global Biopharmaceutical CDMO Service Market spans across various sectors, including pharmaceutical companies, biotechnology companies, and others. Pharmaceutical companies often rely on CDMOs to handle the complex and resource-intensive aspects of drug development and manufacturing. By outsourcing these functions, pharmaceutical companies can focus on their core competencies, such as research and development, while leveraging the specialized skills and infrastructure of CDMOs. This collaboration allows for the efficient production of high-quality biopharmaceuticals, ensuring that they meet regulatory standards and are delivered to market in a timely manner. Biotechnology companies, which are often smaller and more specialized than traditional pharmaceutical companies, also benefit significantly from the services provided by CDMOs. These companies typically focus on cutting-edge research and innovation, developing new therapies and technologies that require advanced manufacturing capabilities. CDMOs offer the expertise and facilities needed to bring these innovative products from the lab to the market, providing support in areas such as process development, scale-up, and regulatory compliance. This partnership enables biotechnology companies to navigate the complex landscape of biopharmaceutical development and commercialization more effectively. Other sectors that utilize CDMO services include academic institutions, government agencies, and non-profit organizations. Academic institutions often engage with CDMOs to translate their research findings into viable biopharmaceutical products. Government agencies may collaborate with CDMOs to develop and manufacture vaccines and other critical therapies, particularly in response to public health emergencies. Non-profit organizations, which may focus on addressing specific health challenges or underserved populations, also benefit from the expertise and capabilities of CDMOs. By partnering with CDMOs, these organizations can advance their missions and bring much-needed treatments to those in need. In summary, the Global Biopharmaceutical CDMO Service Market plays a vital role in supporting the development and manufacturing needs of a diverse range of stakeholders, including pharmaceutical companies, biotechnology companies, academic institutions, government agencies, and non-profit organizations. By providing specialized services and expertise, CDMOs enable these entities to bring innovative biopharmaceutical products to market more efficiently and effectively, ultimately improving patient outcomes and advancing public health.
Global Biopharmaceutical CDMO Service Market Outlook:
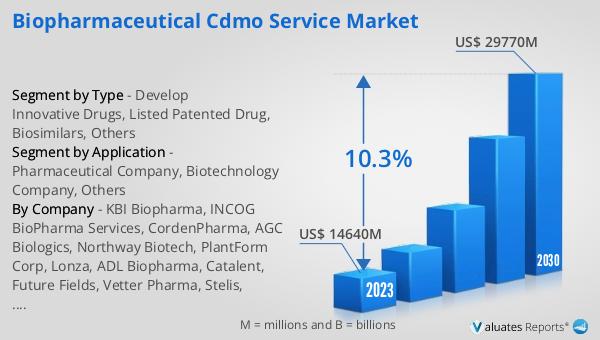
The global Biopharmaceutical CDMO Service market was valued at US$ 14,640 million in 2023 and is anticipated to reach US$ 29,770 million by 2030, witnessing a CAGR of 10.3% during the forecast period 2024-2030. This significant growth reflects the increasing demand for biopharmaceuticals and the critical role that CDMOs play in their development and manufacturing. As the biopharmaceutical industry continues to expand, the need for specialized services provided by CDMOs becomes more pronounced. These organizations offer the expertise, infrastructure, and regulatory knowledge required to bring complex biopharmaceutical products to market. The projected growth of the Biopharmaceutical CDMO Service market underscores the importance of these services in meeting the evolving needs of the biopharmaceutical industry. By partnering with CDMOs, biopharmaceutical companies can navigate the challenges of drug development and manufacturing more effectively, ensuring that innovative therapies reach patients in a timely and cost-efficient manner. The anticipated growth of this market highlights the ongoing demand for high-quality biopharmaceuticals and the essential role that CDMOs play in their production and distribution.
| Report Metric | Details |
| Report Name | Biopharmaceutical CDMO Service Market |
| Accounted market size in 2023 | US$ 14640 million |
| Forecasted market size in 2030 | US$ 29770 million |
| CAGR | 10.3% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | KBI Biopharma, INCOG BioPharma Services, CordenPharma, AGC Biologics, Northway Biotech, PlantForm Corp, Lonza, ADL Biopharma, Catalent, Future Fields, Vetter Pharma, Stelis, Richter-Helm, FUJIFILM Diosynth Biotechnologies, Cambrex, Pfizer CentreOne, Samsung Biologics |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
