What is Global Non-GMO Corn Starch Market?
The Global Non-GMO Corn Starch Market refers to the worldwide industry focused on the production and distribution of corn starch that is derived from non-genetically modified organisms (non-GMO). Corn starch is a versatile carbohydrate extracted from the endosperm of corn kernels and is widely used in various industries due to its thickening, stabilizing, and binding properties. The non-GMO label indicates that the corn used in the production of this starch has not been genetically altered, which is an important consideration for consumers who are increasingly concerned about the health and environmental impacts of genetically modified organisms. This market is driven by the growing demand for clean-label and natural ingredients in food products, as well as the increasing awareness of the potential health benefits associated with non-GMO products. The market encompasses a range of applications, including food and beverage, pharmaceuticals, paper manufacturing, and the chemical industry, among others. As consumers continue to prioritize transparency and sustainability in their purchasing decisions, the Global Non-GMO Corn Starch Market is expected to experience steady growth, with manufacturers focusing on innovation and product development to meet the evolving needs of consumers.
Food Grade, Pharmaceutical Grade, Chemical Grade in the Global Non-GMO Corn Starch Market:
In the Global Non-GMO Corn Starch Market, products are categorized based on their grade, which determines their suitability for different applications. Food grade non-GMO corn starch is specifically processed to meet the safety and quality standards required for use in food products. It is commonly used as a thickening agent in soups, sauces, and gravies, as well as in the production of confectionery items, baked goods, and dairy products. The demand for food-grade non-GMO corn starch is driven by the increasing consumer preference for natural and clean-label ingredients, as well as the growing awareness of the potential health benefits associated with non-GMO products. Pharmaceutical grade non-GMO corn starch, on the other hand, is used in the pharmaceutical industry as an excipient, which is an inactive substance that serves as a vehicle for the active ingredients in medications. It is used to improve the consistency, stability, and bioavailability of drugs, and is also used in the production of tablets, capsules, and other dosage forms. The demand for pharmaceutical-grade non-GMO corn starch is driven by the increasing demand for safe and effective medications, as well as the growing awareness of the potential health benefits associated with non-GMO products. Chemical grade non-GMO corn starch is used in the chemical industry as a raw material for the production of various chemicals, including adhesives, coatings, and biodegradable plastics. It is also used as a binder in the production of paper and textiles, and as a stabilizer in the production of emulsions and suspensions. The demand for chemical-grade non-GMO corn starch is driven by the increasing demand for sustainable and environmentally friendly products, as well as the growing awareness of the potential health benefits associated with non-GMO products. Overall, the Global Non-GMO Corn Starch Market is characterized by a diverse range of applications and a growing demand for natural and sustainable products, with manufacturers focusing on innovation and product development to meet the evolving needs of consumers.
Food, Papermaking, Chemical Industry, Medicine, Others in the Global Non-GMO Corn Starch Market:
The Global Non-GMO Corn Starch Market finds its usage in a variety of areas, each benefiting from the unique properties of non-GMO corn starch. In the food industry, non-GMO corn starch is widely used as a thickening agent in soups, sauces, and gravies, as well as in the production of confectionery items, baked goods, and dairy products. Its ability to provide a smooth texture and enhance the stability of food products makes it a popular choice among food manufacturers. Additionally, the growing consumer preference for natural and clean-label ingredients has further fueled the demand for non-GMO corn starch in the food industry. In the papermaking industry, non-GMO corn starch is used as a binder and coating agent to improve the strength and printability of paper products. Its ability to enhance the surface properties of paper makes it an essential component in the production of high-quality paper and packaging materials. In the chemical industry, non-GMO corn starch is used as a raw material for the production of various chemicals, including adhesives, coatings, and biodegradable plastics. Its biodegradability and renewable nature make it an attractive alternative to petroleum-based chemicals, aligning with the growing demand for sustainable and environmentally friendly products. In the pharmaceutical industry, non-GMO corn starch is used as an excipient in the production of tablets, capsules, and other dosage forms. Its ability to improve the consistency, stability, and bioavailability of drugs makes it a valuable component in the formulation of safe and effective medications. Additionally, the growing awareness of the potential health benefits associated with non-GMO products has further driven the demand for non-GMO corn starch in the pharmaceutical industry. Beyond these industries, non-GMO corn starch is also used in various other applications, including textiles, cosmetics, and personal care products, where its binding, thickening, and stabilizing properties are highly valued. Overall, the Global Non-GMO Corn Starch Market is characterized by a diverse range of applications and a growing demand for natural and sustainable products, with manufacturers focusing on innovation and product development to meet the evolving needs of consumers.
Global Non-GMO Corn Starch Market Outlook:
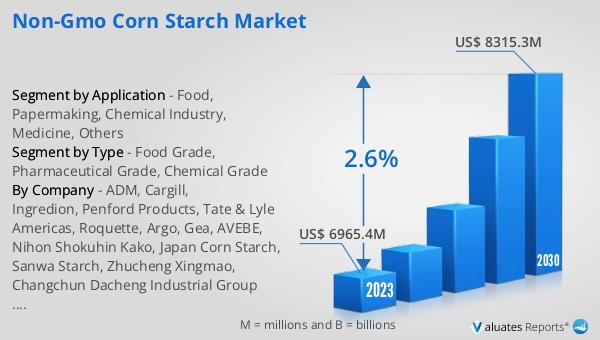
The global market for Non-GMO Corn Starch was valued at $7,295 million in 2024, and it is anticipated to grow to a revised size of $8,709 million by 2031. This growth is expected to occur at a compound annual growth rate (CAGR) of 2.6% during the forecast period. This steady growth reflects the increasing consumer demand for non-GMO products, driven by a growing awareness of the potential health and environmental benefits associated with non-GMO ingredients. As consumers become more health-conscious and environmentally aware, the demand for non-GMO corn starch is expected to rise, leading to increased market opportunities for manufacturers and suppliers. The market's growth is also supported by the expanding applications of non-GMO corn starch in various industries, including food and beverage, pharmaceuticals, paper manufacturing, and the chemical industry. As manufacturers continue to innovate and develop new products to meet the evolving needs of consumers, the Global Non-GMO Corn Starch Market is poised for continued growth and expansion. This growth trajectory highlights the importance of non-GMO corn starch as a key ingredient in a wide range of products, and underscores the need for manufacturers to prioritize sustainability and transparency in their production processes.
| Report Metric | Details |
| Report Name | Non-GMO Corn Starch Market |
| Accounted market size in year | US$ 7295 million |
| Forecasted market size in 2031 | US$ 8709 million |
| CAGR | 2.6% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | ADM, Cargill, Ingredion, Penford Products, Tate & Lyle Americas, Roquette, Argo, Gea, AVEBE, Nihon Shokuhin Kako, Japan Corn Starch, Sanwa Starch, Zhucheng Xingmao, Changchun Dacheng Industrial Group Company, Xiwang Group, Luzhou Group, COPO |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |