What is Global Triptorelin Acetate API Market?
The Global Triptorelin Acetate API Market refers to the worldwide industry focused on the production and distribution of Triptorelin Acetate, an active pharmaceutical ingredient (API) used primarily in the treatment of hormone-sensitive conditions such as prostate cancer, endometriosis, and precocious puberty. This market encompasses various stakeholders, including pharmaceutical companies, research institutions, and healthcare providers, all working together to develop and supply this critical API. Triptorelin Acetate functions as a gonadotropin-releasing hormone (GnRH) agonist, which helps regulate hormone levels in the body, making it a vital component in therapies that require hormone suppression. The market is driven by the increasing prevalence of hormone-related disorders and the growing demand for effective treatments. Additionally, advancements in pharmaceutical research and development have led to improved formulations and delivery methods, further boosting the market's growth. As healthcare systems worldwide continue to prioritize the management of hormone-sensitive conditions, the Global Triptorelin Acetate API Market is expected to expand, offering new opportunities for innovation and collaboration among industry players.
0.95, 0.98, 0.99, Others in the Global Triptorelin Acetate API Market:
In the Global Triptorelin Acetate API Market, the purity levels of the API are crucial, with common specifications being 0.95, 0.98, 0.99, and others. These numbers represent the percentage of purity of the Triptorelin Acetate, which is essential for ensuring the efficacy and safety of the pharmaceutical products in which it is used. A purity level of 0.95 indicates that 95% of the compound is Triptorelin Acetate, with the remaining 5% consisting of impurities or other substances. This level of purity is typically used in applications where the highest level of precision is not critical, or where cost considerations are a significant factor. However, for more sensitive applications, higher purity levels are preferred. A purity level of 0.98 means that 98% of the compound is pure Triptorelin Acetate, offering a higher degree of efficacy and safety. This level is often used in more stringent pharmaceutical applications where the presence of impurities could affect the drug's performance or safety profile. The highest standard, 0.99 purity, is used in the most critical applications, where even the slightest impurity could have significant consequences. This level of purity ensures that the API meets the strictest regulatory standards and provides the highest level of efficacy and safety for patients. Other purity levels may also be available, depending on specific requirements or advancements in purification technology. These variations allow manufacturers to tailor their products to meet the diverse needs of the pharmaceutical industry, balancing cost, efficacy, and safety. The choice of purity level is influenced by several factors, including the intended use of the API, regulatory requirements, and the cost implications of achieving higher purity levels. As the Global Triptorelin Acetate API Market continues to evolve, the demand for different purity levels will likely reflect the growing complexity and specificity of pharmaceutical applications. Manufacturers must remain agile and responsive to these changing demands, ensuring that their products meet the highest standards of quality and safety. This focus on purity is not only a regulatory requirement but also a critical component of maintaining trust and credibility within the pharmaceutical industry. By offering a range of purity levels, the Global Triptorelin Acetate API Market can cater to a wide array of applications, from standard treatments to cutting-edge therapies, supporting the ongoing advancement of healthcare worldwide.
Injection, Others in the Global Triptorelin Acetate API Market:
The Global Triptorelin Acetate API Market plays a significant role in the pharmaceutical industry, particularly in the development and production of injectable medications. Triptorelin Acetate is primarily used in the form of injections to treat hormone-sensitive conditions such as prostate cancer, endometriosis, and precocious puberty. The injectable form of this API allows for precise dosing and rapid absorption into the bloodstream, making it an effective treatment option for conditions that require immediate hormone suppression. Injections are typically administered by healthcare professionals in clinical settings, ensuring that patients receive the correct dosage and monitoring for any adverse reactions. This method of administration is particularly beneficial for patients who require long-term hormone therapy, as it provides a controlled and consistent delivery of the medication. In addition to injections, Triptorelin Acetate is also used in other pharmaceutical formulations, such as implants and depot injections, which offer extended-release options for patients. These formulations are designed to provide a sustained release of the medication over a specified period, reducing the frequency of administration and improving patient compliance. The versatility of Triptorelin Acetate in various formulations highlights its importance in the treatment of hormone-sensitive conditions and underscores the need for continued research and development in this area. As the Global Triptorelin Acetate API Market continues to grow, the demand for innovative delivery methods and formulations will likely increase, driving further advancements in the field. This ongoing innovation is essential for meeting the diverse needs of patients and healthcare providers, ensuring that Triptorelin Acetate remains a vital component of hormone therapy worldwide. By offering a range of administration options, the Global Triptorelin Acetate API Market can support the development of personalized treatment plans that cater to the unique needs of each patient, ultimately improving outcomes and enhancing the quality of life for those affected by hormone-sensitive conditions.
Global Triptorelin Acetate API Market Outlook:
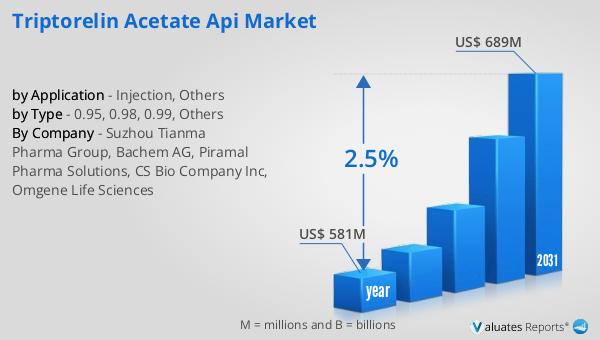
The global market for Triptorelin Acetate API was valued at $581 million in 2024 and is anticipated to grow to a revised size of $689 million by 2031, reflecting a compound annual growth rate (CAGR) of 2.5% over the forecast period. This growth is indicative of the increasing demand for Triptorelin Acetate in the treatment of hormone-sensitive conditions, driven by the rising prevalence of such disorders and the ongoing advancements in pharmaceutical research and development. In comparison, the global pharmaceutical market was valued at $1,475 billion in 2022, with a projected CAGR of 5% over the next six years. This broader market growth highlights the expanding opportunities within the pharmaceutical industry, as companies continue to innovate and develop new treatments to address a wide range of health conditions. Meanwhile, the chemical drug market, a subset of the pharmaceutical industry, was estimated to grow from $1,005 billion in 2018 to $1,094 billion in 2022. This growth underscores the importance of chemical drugs, including APIs like Triptorelin Acetate, in the development of effective and safe medications. As the Global Triptorelin Acetate API Market continues to evolve, it will play a crucial role in supporting the broader pharmaceutical industry, contributing to the development of innovative treatments and improving patient outcomes worldwide.
| Report Metric | Details |
| Report Name | Triptorelin Acetate API Market |
| Accounted market size in year | US$ 581 million |
| Forecasted market size in 2031 | US$ 689 million |
| CAGR | 2.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Suzhou Tianma Pharma Group, Bachem AG, Piramal Pharma Solutions, CS Bio Company Inc, Omgene Life Sciences |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |