What is Global Implanted Urinary Incontinence Device Market?
The Global Implanted Urinary Incontinence Device Market is a specialized sector within the medical device industry, focusing on products designed to manage urinary incontinence. This condition, which affects millions worldwide, leads to the involuntary leakage of urine, causing significant distress and impacting the quality of life. The market encompasses a range of devices implanted into the body to either restore the normal function of the urinary system or to replace it partially. These devices are critical for patients who have not found relief through conventional treatments such as medication or pelvic floor exercises. As the global population ages and the prevalence of conditions like obesity and diabetes increases—both of which are risk factors for urinary incontinence—the demand for these implanted devices is expected to grow. Innovations in device design, materials, and the minimally invasive techniques used to implant them are also driving the market forward, making treatments more effective and accessible to a broader range of patients. This sector is not just about providing medical devices; it's about restoring dignity and improving the quality of life for those affected by urinary incontinence.
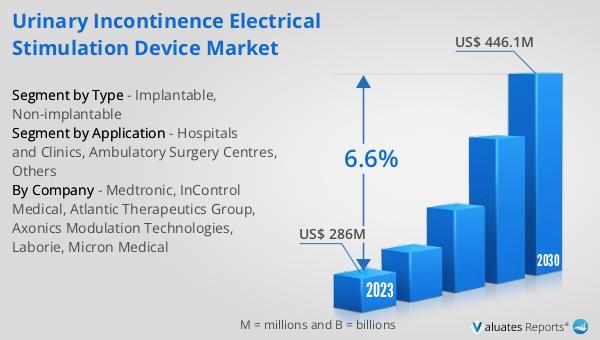
Artificial Urinary Sphincters, Electrical Stimulation Devices, Others in the Global Implanted Urinary Incontinence Device Market:
Diving deeper into the Global Implanted Urinary Incontinence Device Market, we find it segmented into various types of devices, each designed to address different aspects and causes of urinary incontinence. Artificial Urinary Sphincters (AUS) are among the most common devices, designed to mimic the function of a healthy urinary sphincter. This device is particularly beneficial for patients with severe urinary incontinence, providing them with the ability to control urination manually. Electrical Stimulation Devices represent another segment, offering a less invasive option. These devices work by stimulating the nerves responsible for bladder control, helping to strengthen the muscles involved in urination and thus reducing incidents of leakage. Other types of devices within this market include urethral inserts and balloon therapy devices, each catering to specific patient needs and conditions. The development and refinement of these devices are ongoing, with research focused on improving efficacy, reducing the risk of infection, and minimizing the invasiveness of the implantation procedure. As technology advances, so too does the potential for these devices to offer relief and a return to normalcy for individuals suffering from urinary incontinence. The market for these devices is not just about the technology itself but about the hope and improved quality of life they offer to patients.
Hospitals and Clinics, Ambulatory Surgery Centres, Others in the Global Implanted Urinary Incontinence Device Market:
The usage of the Global Implanted Urinary Incontinence Device Market spans various healthcare settings, reflecting the widespread need for effective urinary incontinence management solutions. Hospitals and clinics represent the primary venues where these devices are implanted, given the need for specialized surgical expertise. These settings offer the advantage of comprehensive care, from diagnosis to post-operative follow-up, ensuring patients receive the full spectrum of treatment and support. Ambulatory Surgery Centers (ASCs) have also emerged as key players in this market, providing a more cost-effective, convenient option for patients. ASCs specialize in outpatient procedures, including the implantation of urinary incontinence devices, allowing patients to return home the same day. This not only reduces healthcare costs but also minimizes the disruption to patients' lives. Other healthcare settings, such as specialized urology centers, also play a role in the delivery of these treatments, offering focused expertise in urinary health. The choice of setting depends on various factors, including the complexity of the patient's condition, the type of device being implanted, and the patient's overall health and preferences. Regardless of the setting, the goal remains the same: to provide effective, compassionate care that addresses the physical and emotional aspects of urinary incontinence.
Global Implanted Urinary Incontinence Device Market Outlook:
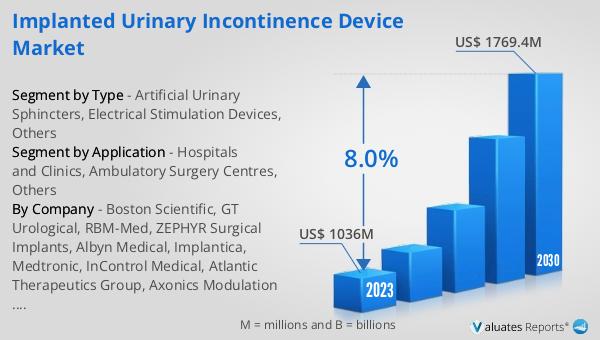
The market outlook for the Global Implanted Urinary Incontinence Device Market presents a promising future, with the sector's value estimated at US$ 1036 million in 2023, and projections suggesting it will rise to US$ 1769.4 million by 2030. This growth trajectory, characterized by a Compound Annual Growth Rate (CAGR) of 8.0% during the forecast period from 2024 to 2030, underscores the increasing demand and ongoing innovation within this niche of the medical device industry. This expansion is part of a broader trend within the global market for medical devices, which itself is estimated to be worth US$ 603 billion in 2023, with expectations of growing at a CAGR of 5% over the next six years. These figures highlight the significant role that implanted urinary incontinence devices play in the larger healthcare landscape, driven by a growing recognition of the need for advanced, effective treatments for urinary incontinence. As research continues and technology advances, the market is set to offer new solutions to improve the lives of those affected by this condition, reflecting both the economic value and the profound impact on patient quality of life.
| Report Metric | Details |
| Report Name | Implanted Urinary Incontinence Device Market |
| Accounted market size in 2023 | US$ 1036 million |
| Forecasted market size in 2030 | US$ 1769.4 million |
| CAGR | 8.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Boston Scientific, GT Urological, RBM-Med, ZEPHYR Surgical Implants, Albyn Medical, Implantica, Medtronic, InControl Medical, Atlantic Therapeutics Group, Axonics Modulation Technologies, Laborie, Micron Medical |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |