What is Global Contract Research Organization (CRO) Services Market?
The Global Contract Research Organization (CRO) Services Market is a dynamic and essential component of the healthcare and pharmaceutical industries. CROs are specialized service providers that offer a wide range of research and development services to pharmaceutical, biotechnology, and medical device companies. These services include preclinical research, clinical trials, regulatory support, data management, and more. The primary goal of CROs is to assist these companies in bringing new drugs and medical devices to market more efficiently and cost-effectively. By outsourcing these critical functions to CROs, companies can focus on their core competencies while leveraging the expertise and resources of CROs to navigate the complex and highly regulated landscape of drug and device development. The global CRO market is driven by the increasing demand for innovative therapies, the rising complexity of clinical trials, and the need for cost containment in drug development. As a result, CROs play a crucial role in accelerating the development of new treatments and improving patient outcomes worldwide.
Preclinical CRO, Clinical Trial CRO in the Global Contract Research Organization (CRO) Services Market:
Preclinical CROs and Clinical Trial CROs are two vital segments within the Global Contract Research Organization (CRO) Services Market, each serving distinct but complementary roles in the drug development process. Preclinical CROs focus on the early stages of research, providing services that include in vitro and in vivo testing, toxicology studies, pharmacokinetics, and pharmacodynamics. These services are crucial for determining the safety and efficacy of a drug candidate before it progresses to human trials. Preclinical CROs employ advanced technologies and methodologies to assess the biological activity of compounds, identify potential side effects, and establish safe dosage levels. By outsourcing preclinical research to specialized CROs, pharmaceutical and biotechnology companies can benefit from their expertise, state-of-the-art facilities, and adherence to regulatory standards, ultimately accelerating the transition from discovery to clinical development. On the other hand, Clinical Trial CROs are involved in the later stages of drug development, managing and conducting clinical trials that evaluate the safety and efficacy of new drugs and medical devices in human subjects. These CROs provide a comprehensive range of services, including study design, patient recruitment, site management, data collection, statistical analysis, and regulatory compliance. Clinical Trial CROs play a critical role in ensuring that clinical trials are conducted efficiently, ethically, and in accordance with regulatory requirements. They help sponsors navigate the complexities of trial design and execution, manage the logistical challenges of multi-site studies, and ensure the integrity and reliability of trial data. By leveraging the expertise and infrastructure of Clinical Trial CROs, sponsors can reduce the time and cost associated with bringing new therapies to market, while also minimizing the risks of trial delays and failures. The integration of preclinical and clinical trial services within the CRO market allows for a seamless transition from early-stage research to human trials, facilitating a more streamlined and efficient drug development process. This integration is particularly important in the context of increasingly complex and globalized clinical trials, which require sophisticated project management and coordination across multiple sites and regulatory jurisdictions. CROs with capabilities in both preclinical and clinical trial services are well-positioned to offer end-to-end solutions that address the full spectrum of drug development needs, from initial discovery through to regulatory approval and commercialization. Moreover, the growing trend towards personalized medicine and the development of targeted therapies has increased the demand for specialized CRO services that can support the unique requirements of these innovative approaches. Preclinical CROs are increasingly involved in the development of biomarkers and companion diagnostics, which are essential for identifying patient populations that are most likely to benefit from specific treatments. Similarly, Clinical Trial CROs are adapting to the challenges of conducting trials for personalized therapies, which often involve smaller patient populations and more complex trial designs. In summary, Preclinical and Clinical Trial CROs are indispensable partners in the drug development process, providing the expertise, resources, and infrastructure needed to bring new therapies to market efficiently and effectively. Their role in the Global Contract Research Organization (CRO) Services Market is critical to advancing medical innovation and improving patient outcomes worldwide.
Pharmaceutical Industries, Biotechnology Industries, Medical Device Industries in the Global Contract Research Organization (CRO) Services Market:
The Global Contract Research Organization (CRO) Services Market plays a pivotal role in the pharmaceutical, biotechnology, and medical device industries by providing essential services that support the development and commercialization of new products. In the pharmaceutical industry, CROs are instrumental in conducting preclinical and clinical research, which are critical stages in the drug development process. Pharmaceutical companies rely on CROs to manage the complexities of clinical trials, including patient recruitment, site management, data collection, and regulatory compliance. By outsourcing these functions to CROs, pharmaceutical companies can focus on their core competencies, reduce development costs, and accelerate the time-to-market for new drugs. CROs also provide valuable expertise in navigating the regulatory landscape, ensuring that clinical trials are conducted in accordance with international standards and guidelines. In the biotechnology industry, CROs offer specialized services that cater to the unique needs of biotech companies, which often focus on developing innovative therapies and biologics. Biotechnology companies benefit from the advanced scientific expertise and cutting-edge technologies provided by CROs, which are essential for conducting complex preclinical studies and clinical trials. CROs also assist biotech companies in overcoming the challenges associated with scaling up production and navigating the regulatory pathways for novel therapies. By partnering with CROs, biotechnology companies can leverage their resources and expertise to bring groundbreaking treatments to market more efficiently and effectively. The medical device industry also relies heavily on CROs for support in the development and commercialization of new devices. CROs provide a range of services, including regulatory consulting, clinical trial management, and post-market surveillance, which are critical for ensuring the safety and efficacy of medical devices. Medical device companies face unique challenges in navigating the regulatory landscape, as the requirements for device approval can vary significantly across different regions. CROs help these companies navigate the complex regulatory environment, ensuring that their products meet the necessary standards and requirements for market entry. Additionally, CROs assist in the design and execution of clinical trials for medical devices, which often require specialized expertise and infrastructure. Overall, the Global Contract Research Organization (CRO) Services Market is an indispensable partner for pharmaceutical, biotechnology, and medical device industries, providing the expertise, resources, and infrastructure needed to bring new products to market efficiently and effectively. By outsourcing critical research and development functions to CROs, these industries can focus on their core competencies, reduce costs, and accelerate the development of innovative therapies and devices that improve patient outcomes worldwide.
Global Contract Research Organization (CRO) Services Market Outlook:
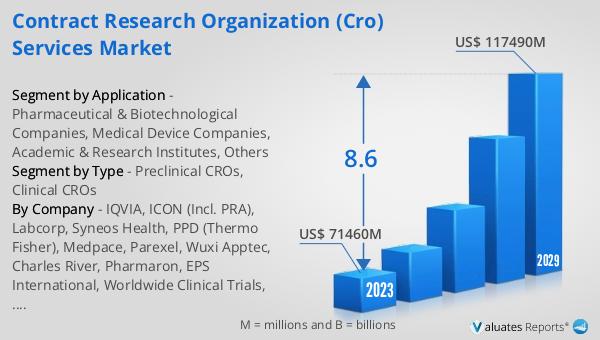
The outlook for the Global Contract Research Organization (CRO) Services Market indicates a robust growth trajectory, with projections suggesting an increase from $71,460 million in 2024 to $117,490 million by 2030, reflecting a Compound Annual Growth Rate (CAGR) of 8.6% over the forecast period. This growth is driven by the increasing demand for outsourced research and development services, as pharmaceutical and biotechnology companies seek to optimize their drug development processes and reduce costs. In comparison, the global pharmaceutical market, valued at $1,475 billion in 2022, is expected to grow at a CAGR of 5% over the next six years, highlighting the faster growth rate of the CRO market. Meanwhile, the chemical drug market is projected to expand from $1,005 billion in 2018 to $1,094 billion by 2022. This data underscores the significant role that CROs play in the broader pharmaceutical and healthcare landscape, as they provide essential services that enable companies to bring new therapies and medical devices to market more efficiently and effectively. The continued growth of the CRO market is a testament to the increasing reliance on outsourced research and development services, as companies seek to navigate the complexities of drug and device development in an increasingly competitive and regulated environment.
| Report Metric | Details |
| Report Name | Contract Research Organization (CRO) Services Market |
| Accounted market size in 2024 | US$ 71460 million |
| Forecasted market size in 2030 | US$ 117490 million |
| CAGR | 8.6 |
| Base Year | 2024 |
| Forecasted years | 2025 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Charles River Laboratories, ICON, Parexel, PPD, Quintiles, Covance, PRA Health Sciences, Syneos Health, Wuxi AppTec, Medpace, Catalent, Aenova, FAMAR, Vetter, DPT Laboratories, Recipharm, NextPharma, Aesica |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
