What is Global Clinical Trial Supply Services Market?
The Global Clinical Trial Supply Services Market is a crucial component of the pharmaceutical and biotechnology industries, providing essential support for the development and testing of new drugs and therapies. This market encompasses a wide range of services, including the manufacturing, packaging, labeling, logistics, and distribution of clinical trial materials. These services ensure that clinical trials are conducted efficiently and effectively, with the right materials delivered to the right place at the right time. The market is driven by the increasing complexity of clinical trials, the globalization of research activities, and the growing demand for personalized medicine. As pharmaceutical companies and research organizations strive to bring new treatments to market faster, the need for reliable and efficient clinical trial supply services continues to grow. This market plays a vital role in ensuring that clinical trials are conducted smoothly, helping to accelerate the development of new therapies and improve patient outcomes worldwide.
Manufacturing, Packaging and Labeling, Logistics and Distribution in the Global Clinical Trial Supply Services Market:
Manufacturing, packaging, labeling, logistics, and distribution are integral components of the Global Clinical Trial Supply Services Market, each playing a critical role in the successful execution of clinical trials. Manufacturing involves the production of investigational drugs and placebos in compliance with stringent regulatory standards. This process requires precision and adherence to Good Manufacturing Practices (GMP) to ensure the safety and efficacy of the trial materials. Once manufactured, these materials must be packaged in a manner that maintains their integrity and stability throughout the trial. Packaging solutions are designed to protect the products from environmental factors and ensure that they are delivered in optimal condition. Labeling is another crucial aspect, as it provides essential information about the trial materials, including dosage instructions, storage conditions, and expiration dates. Accurate labeling is vital to ensure compliance with regulatory requirements and to facilitate the proper administration of the trial materials by healthcare professionals. Logistics and distribution involve the transportation and delivery of clinical trial materials to various trial sites, often located in different countries. This requires careful planning and coordination to ensure that the materials are delivered on time and in the right quantities. The logistics process must also account for temperature-sensitive products, which require specialized handling and storage to maintain their efficacy. Distribution networks must be robust and flexible to accommodate the dynamic nature of clinical trials, which may involve changes in trial sites or patient recruitment numbers. Overall, the seamless integration of manufacturing, packaging, labeling, logistics, and distribution is essential to the success of clinical trials, ensuring that they are conducted efficiently and that the trial materials reach the intended recipients without delay.
Oncology, Cardiovascular Diseases, Respiratory Diseases, CNS And Mental Disorders, Dermatology, Blood Disorders, Infectious Diseases, Others in the Global Clinical Trial Supply Services Market:
The Global Clinical Trial Supply Services Market plays a pivotal role in the development of treatments for a wide range of medical conditions, including oncology, cardiovascular diseases, respiratory diseases, CNS and mental disorders, dermatology, blood disorders, infectious diseases, and others. In oncology, clinical trials are essential for testing new cancer therapies and improving existing treatments. The supply services market ensures that investigational drugs are delivered to trial sites promptly, enabling researchers to evaluate their efficacy and safety in cancer patients. For cardiovascular diseases, clinical trials are crucial for developing new medications and interventions to manage heart-related conditions. The supply services market supports these trials by providing the necessary materials and ensuring their timely delivery to trial sites. In the field of respiratory diseases, clinical trials help in the development of new treatments for conditions such as asthma and chronic obstructive pulmonary disease (COPD). The supply services market ensures that trial materials are available when needed, facilitating the smooth conduct of these trials. CNS and mental disorders, including depression, anxiety, and schizophrenia, require extensive clinical research to develop effective treatments. The supply services market plays a vital role in supporting these trials by providing the necessary materials and ensuring their accurate labeling and distribution. Dermatology trials focus on developing new treatments for skin conditions such as psoriasis and eczema. The supply services market ensures that trial materials are packaged and labeled correctly, enabling researchers to conduct these trials efficiently. Blood disorders, including hemophilia and anemia, require clinical trials to test new therapies and improve patient outcomes. The supply services market supports these trials by ensuring the timely delivery of trial materials to research sites. Infectious diseases, such as HIV/AIDS and hepatitis, require ongoing clinical research to develop new treatments and vaccines. The supply services market plays a crucial role in supporting these trials by providing the necessary materials and ensuring their proper distribution. Overall, the Global Clinical Trial Supply Services Market is essential for the successful execution of clinical trials across various therapeutic areas, helping to advance medical research and improve patient care.
Global Clinical Trial Supply Services Market Outlook:
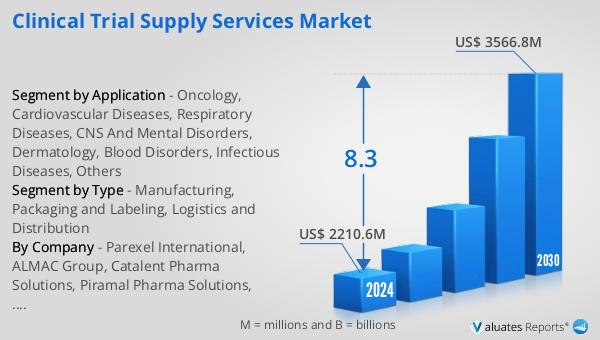
The outlook for the Global Clinical Trial Supply Services Market indicates a promising growth trajectory. The market is anticipated to expand from $2,210.6 million in 2024 to $3,566.8 million by 2030, reflecting a Compound Annual Growth Rate (CAGR) of 8.3% over the forecast period. This growth is driven by the increasing demand for efficient and reliable clinical trial supply services, as pharmaceutical companies and research organizations strive to accelerate the development of new therapies. The market's expansion is also supported by the globalization of clinical trials, which necessitates robust supply chain solutions to manage the distribution of trial materials across multiple regions. In China, the top five manufacturers hold a significant production share, accounting for over 70% of the market. This concentration of production capacity highlights the importance of strategic partnerships and collaborations in the clinical trial supply services market. As the market continues to grow, companies will need to invest in advanced technologies and infrastructure to enhance their supply chain capabilities and meet the evolving needs of the pharmaceutical and biotechnology industries. The Global Clinical Trial Supply Services Market is poised for significant growth, driven by the increasing complexity of clinical trials and the growing demand for personalized medicine.
| Report Metric | Details |
| Report Name | Clinical Trial Supply Services Market |
| Accounted market size in 2024 | US$ 2210.6 million |
| Forecasted market size in 2030 | US$ 3566.8 million |
| CAGR | 8.3 |
| Base Year | 2024 |
| Forecasted years | 2025 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Parexel International, ALMAC Group, Catalent Pharma Solutions, Piramal Pharma Solutions, Shertech Manufacturing, Thermo Fisher Scientific, PCI Services, Patheon, Inc., Sharp Packaging Services, Biocair, Movianto, Klifo A/S. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
