What is Global Clinical Trial Supply Management Market?
The Global Clinical Trial Supply Management Market is a crucial component of the pharmaceutical and healthcare industries, focusing on the efficient management of supplies necessary for clinical trials. Clinical trials are essential for the development of new drugs, therapies, and medical devices, and they require a well-organized supply chain to ensure that all necessary materials are available when and where they are needed. This market encompasses a range of activities, including the sourcing, storage, distribution, and management of clinical trial materials such as drugs, placebos, and medical devices. The goal is to ensure that clinical trials are conducted smoothly, without delays or interruptions, which can be costly and impact the development timeline of new treatments. The market is driven by the increasing complexity of clinical trials, globalization of research activities, and the need for compliance with stringent regulatory requirements. As clinical trials become more global, the demand for efficient supply chain management solutions continues to grow, making this market an essential part of the healthcare ecosystem.
Software, Services in the Global Clinical Trial Supply Management Market:
In the Global Clinical Trial Supply Management Market, software and services play a pivotal role in ensuring the seamless execution of clinical trials. Software solutions are designed to streamline various aspects of supply chain management, from planning and forecasting to inventory management and distribution. These solutions often include features such as real-time tracking, data analytics, and reporting capabilities, which help organizations optimize their supply chain operations and make informed decisions. For instance, software can predict demand for trial materials, manage inventory levels, and ensure timely delivery to trial sites, reducing the risk of delays and disruptions. Additionally, software solutions often integrate with other systems, such as electronic data capture (EDC) and clinical trial management systems (CTMS), to provide a comprehensive view of the trial process. On the services side, companies in the clinical trial supply management market offer a range of support services to assist pharmaceutical companies and research organizations in managing their supply chains. These services can include logistics and distribution, packaging and labeling, and regulatory compliance support. Logistics and distribution services ensure that trial materials are transported safely and efficiently to trial sites, often involving complex coordination across multiple countries and regions. Packaging and labeling services are crucial for maintaining the integrity and compliance of trial materials, as they must adhere to specific regulatory requirements and ensure patient safety. Regulatory compliance support services help organizations navigate the complex landscape of global regulations, ensuring that all trial materials meet the necessary standards and requirements. Furthermore, the integration of advanced technologies such as artificial intelligence (AI) and blockchain is transforming the clinical trial supply management market. AI can enhance forecasting accuracy, optimize inventory management, and improve decision-making processes, while blockchain technology offers increased transparency and security in the supply chain. These innovations are helping organizations address challenges such as supply chain disruptions, counterfeit products, and data integrity issues, ultimately improving the efficiency and reliability of clinical trials. Overall, the combination of software and services in the Global Clinical Trial Supply Management Market is essential for ensuring the successful execution of clinical trials. By leveraging advanced technologies and expert support services, organizations can optimize their supply chain operations, reduce costs, and accelerate the development of new treatments and therapies. As the demand for efficient clinical trial supply management solutions continues to grow, the market is expected to evolve and expand, offering new opportunities for innovation and collaboration.
Pharmaceutical, Biologics, Medical Device, Others in the Global Clinical Trial Supply Management Market:
The Global Clinical Trial Supply Management Market plays a vital role in various areas, including pharmaceuticals, biologics, medical devices, and others. In the pharmaceutical sector, clinical trial supply management is crucial for the development of new drugs and therapies. Pharmaceutical companies rely on efficient supply chain management to ensure that trial materials are available at the right time and place, minimizing delays and disruptions. This involves coordinating the sourcing, storage, and distribution of drugs and placebos, as well as managing inventory levels and ensuring compliance with regulatory requirements. By optimizing their supply chain operations, pharmaceutical companies can accelerate the development process and bring new treatments to market more quickly. In the biologics sector, clinical trial supply management is essential for the development of complex biologic products, such as vaccines, monoclonal antibodies, and gene therapies. These products often require specialized handling and storage conditions, making supply chain management even more critical. Companies in this sector must ensure that trial materials are transported and stored under the appropriate conditions to maintain their integrity and efficacy. This involves coordinating logistics and distribution, as well as managing packaging and labeling to ensure compliance with regulatory standards. By leveraging advanced supply chain management solutions, biologics companies can overcome the unique challenges associated with developing and testing these complex products. The medical device sector also relies on clinical trial supply management to support the development and testing of new devices. This involves managing the sourcing, storage, and distribution of trial materials, as well as ensuring compliance with regulatory requirements. Medical device companies must coordinate logistics and distribution to ensure that trial materials are available at the right time and place, minimizing delays and disruptions. Additionally, they must manage packaging and labeling to ensure compliance with regulatory standards and maintain the integrity of trial materials. By optimizing their supply chain operations, medical device companies can accelerate the development process and bring new devices to market more quickly. In addition to pharmaceuticals, biologics, and medical devices, the Global Clinical Trial Supply Management Market also supports other areas, such as diagnostics and nutraceuticals. In these sectors, efficient supply chain management is essential for ensuring the availability and integrity of trial materials, as well as compliance with regulatory requirements. By leveraging advanced supply chain management solutions, companies in these sectors can optimize their operations, reduce costs, and accelerate the development of new products and therapies. Overall, the Global Clinical Trial Supply Management Market is a critical component of the healthcare ecosystem, supporting the development and testing of new treatments and technologies across a wide range of areas.
Global Clinical Trial Supply Management Market Outlook:
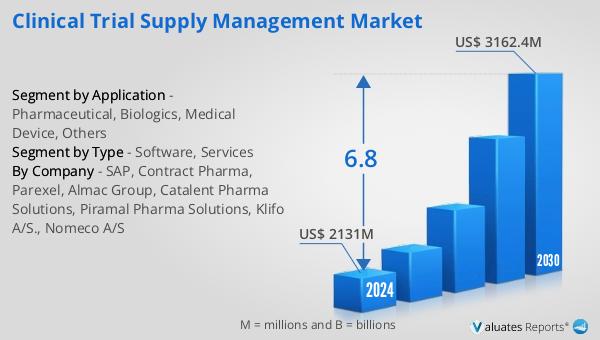
The outlook for the Global Clinical Trial Supply Management Market indicates a promising growth trajectory over the coming years. The market is anticipated to expand from a valuation of $2,131 million in 2024 to approximately $3,162.4 million by 2030. This growth is expected to occur at a compound annual growth rate (CAGR) of 6.8% during the forecast period. This upward trend reflects the increasing demand for efficient supply chain management solutions in the clinical trial sector, driven by the growing complexity and globalization of clinical trials. As pharmaceutical companies and research organizations continue to expand their research activities across multiple regions, the need for robust supply chain management becomes more critical. The market's growth is also supported by advancements in technology, such as the integration of artificial intelligence and blockchain, which are enhancing the efficiency and reliability of supply chain operations. Additionally, the increasing focus on regulatory compliance and patient safety is driving the demand for specialized services and solutions in the clinical trial supply management market. Overall, the market outlook suggests a positive trajectory, with significant opportunities for innovation and growth in the coming years.
| Report Metric | Details |
| Report Name | Clinical Trial Supply Management Market |
| Accounted market size in 2024 | US$ 2131 million |
| Forecasted market size in 2030 | US$ 3162.4 million |
| CAGR | 6.8 |
| Base Year | 2024 |
| Forecasted years | 2025 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | SAP, Contract Pharma, Parexel, Almac Group, Catalent Pharma Solutions, Piramal Pharma Solutions, Klifo A/S., Nomeco A/S |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
