What is Global Innovative Drug Commercialization Service Platform Market?
The Global Innovative Drug Commercialization Service Platform Market is a rapidly evolving sector that focuses on the development, marketing, and distribution of new pharmaceutical drugs on a global scale. This market encompasses a wide range of services, including research and development, clinical trials, regulatory compliance, marketing strategies, and distribution channels. The primary goal of these platforms is to streamline the process of bringing innovative drugs to market, ensuring they reach patients in need as quickly and efficiently as possible. These platforms leverage advanced technologies and data analytics to optimize every stage of the drug commercialization process, from initial research to post-market surveillance. By providing a comprehensive suite of services, these platforms help pharmaceutical companies navigate the complex and highly regulated landscape of drug development and commercialization, ultimately improving patient outcomes and driving growth in the pharmaceutical industry.
Local Deployment, Cloud Based in the Global Innovative Drug Commercialization Service Platform Market:
Local deployment and cloud-based solutions are two primary models for implementing Global Innovative Drug Commercialization Service Platforms. Local deployment refers to the installation and operation of software and services on the premises of the pharmaceutical company. This model offers several advantages, including greater control over data security, customization options, and potentially lower long-term costs. Companies with robust IT infrastructure and resources may prefer local deployment to maintain direct oversight of their operations and ensure compliance with stringent regulatory requirements. However, local deployment can also present challenges, such as higher upfront costs, ongoing maintenance, and the need for specialized IT personnel to manage the system. On the other hand, cloud-based solutions offer a more flexible and scalable approach to drug commercialization. These platforms are hosted on remote servers and accessed via the internet, allowing companies to leverage advanced technologies without the need for significant upfront investment in hardware and software. Cloud-based solutions provide several benefits, including reduced IT overhead, automatic updates, and the ability to scale resources up or down based on demand. This model is particularly advantageous for small and medium-sized enterprises (SMEs) that may lack the resources to invest in extensive IT infrastructure. Additionally, cloud-based platforms often come with built-in security features and compliance certifications, ensuring that sensitive data is protected and regulatory requirements are met. One of the key advantages of cloud-based solutions is their ability to facilitate collaboration and data sharing across geographically dispersed teams. In the context of global drug commercialization, this is particularly important, as it enables seamless communication and coordination between research and development teams, regulatory bodies, marketing departments, and distribution partners. Cloud-based platforms also support advanced data analytics and machine learning capabilities, which can help companies identify trends, optimize processes, and make data-driven decisions. For example, predictive analytics can be used to forecast market demand, identify potential risks, and develop targeted marketing strategies. Despite the numerous benefits of cloud-based solutions, there are also potential drawbacks to consider. Data security and privacy concerns are often cited as major challenges, particularly in the highly regulated pharmaceutical industry. Companies must ensure that their cloud service providers adhere to strict security protocols and comply with relevant regulations, such as the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA). Additionally, reliance on third-party providers can introduce risks related to service availability and vendor lock-in, where companies become dependent on a single provider for their critical operations. In conclusion, both local deployment and cloud-based solutions offer unique advantages and challenges for implementing Global Innovative Drug Commercialization Service Platforms. The choice between these models depends on various factors, including the size and resources of the company, regulatory requirements, and specific business needs. By carefully evaluating these factors, pharmaceutical companies can select the most appropriate deployment model to support their drug commercialization efforts and drive success in the competitive global market.
Large Enterprise, Medium-Sized Enterprise, Small Companies in the Global Innovative Drug Commercialization Service Platform Market:
The usage of Global Innovative Drug Commercialization Service Platforms varies significantly across large enterprises, medium-sized enterprises, and small companies, each with its unique set of needs and challenges. Large enterprises, with their extensive resources and global reach, often leverage these platforms to streamline their complex drug development and commercialization processes. These companies typically have multiple research and development (R&D) centers, clinical trial sites, and marketing teams spread across different regions. By utilizing a comprehensive service platform, large enterprises can ensure seamless coordination and communication between these disparate teams, leading to more efficient and effective drug development and commercialization. Additionally, these platforms provide advanced data analytics and machine learning capabilities, enabling large enterprises to make data-driven decisions, optimize their processes, and stay ahead of the competition. Medium-sized enterprises, on the other hand, may not have the same level of resources as their larger counterparts but still require robust solutions to support their drug commercialization efforts. These companies often operate in niche markets or focus on specific therapeutic areas, making it essential to have a flexible and scalable platform that can adapt to their unique needs. Cloud-based solutions are particularly advantageous for medium-sized enterprises, as they offer the necessary scalability and flexibility without the need for significant upfront investment in IT infrastructure. By leveraging cloud-based platforms, medium-sized enterprises can access advanced technologies and data analytics capabilities, enabling them to compete effectively in the global market. Furthermore, these platforms can help medium-sized enterprises navigate the complex regulatory landscape, ensuring compliance with local and international regulations. Small companies, including startups and early-stage biotech firms, face unique challenges in the drug commercialization process. Limited resources, lack of expertise, and stringent regulatory requirements can make it difficult for these companies to bring their innovative drugs to market. Global Innovative Drug Commercialization Service Platforms can provide invaluable support to small companies by offering a comprehensive suite of services tailored to their specific needs. Cloud-based solutions are particularly beneficial for small companies, as they provide access to advanced technologies and data analytics capabilities without the need for significant upfront investment. Additionally, these platforms often come with built-in compliance features, helping small companies navigate the complex regulatory landscape and ensure their products meet all necessary requirements. In summary, Global Innovative Drug Commercialization Service Platforms play a crucial role in supporting the drug development and commercialization efforts of large enterprises, medium-sized enterprises, and small companies. By providing a comprehensive suite of services, advanced data analytics capabilities, and flexible deployment options, these platforms help companies of all sizes navigate the complex and highly regulated pharmaceutical industry. Whether through local deployment or cloud-based solutions, these platforms enable companies to optimize their processes, make data-driven decisions, and ultimately bring innovative drugs to market more efficiently and effectively.
Global Innovative Drug Commercialization Service Platform Market Outlook:
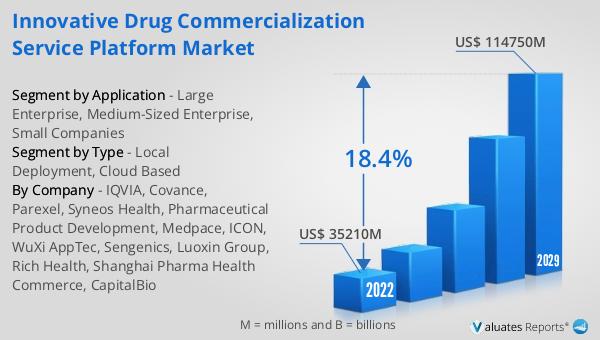
The global Innovative Drug Commercialization Service Platform market was valued at US$ 35,210 million in 2023 and is anticipated to reach US$ 114,750 million by 2030, witnessing a compound annual growth rate (CAGR) of 18.4% during the forecast period from 2024 to 2030. This significant growth reflects the increasing demand for efficient and effective drug commercialization solutions in the pharmaceutical industry. As companies strive to bring innovative drugs to market more quickly and efficiently, the need for comprehensive service platforms that can streamline the entire process from research and development to post-market surveillance is becoming increasingly important. These platforms leverage advanced technologies and data analytics to optimize every stage of the drug commercialization process, ensuring that new drugs reach patients in need as quickly and efficiently as possible. The projected growth of the market underscores the critical role these platforms play in driving innovation and improving patient outcomes in the pharmaceutical industry.
| Report Metric | Details |
| Report Name | Innovative Drug Commercialization Service Platform Market |
| Accounted market size in 2023 | US$ 35210 million |
| Forecasted market size in 2030 | US$ 114750 million |
| CAGR | 18.4% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | IQVIA, Covance, Parexel, Syneos Health, Pharmaceutical Product Development, Medpace, ICON, WuXi AppTec, Sengenics, Luoxin Group, Rich Health, Shanghai Pharma Health Commerce, CapitalBio |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
