What is Global Inline Pharmaceutical Label Inspection Market?
The Global Inline Pharmaceutical Label Inspection Market is a specialized segment within the pharmaceutical industry that focuses on ensuring the accuracy and quality of labels on pharmaceutical products. This market involves the use of advanced technologies and systems to inspect labels inline during the production process, ensuring that they meet regulatory standards and are free from errors. These inspections are crucial because incorrect labeling can lead to serious health risks, regulatory penalties, and loss of consumer trust. The market includes various types of inspection systems such as barcode verification, tag presence detection, and other advanced technologies. These systems are designed to detect issues like misprints, incorrect information, and missing labels, thereby ensuring that only correctly labeled products reach the market. The demand for inline pharmaceutical label inspection systems is driven by the increasing need for compliance with stringent regulatory requirements and the growing focus on patient safety. As pharmaceutical companies strive to maintain high standards of quality and safety, the adoption of these inspection systems is becoming increasingly important.
Barcode Verification System, Tag Presence Detection System, Other in the Global Inline Pharmaceutical Label Inspection Market:
The Barcode Verification System is a critical component of the Global Inline Pharmaceutical Label Inspection Market. This system ensures that the barcodes on pharmaceutical labels are accurate and scannable. Barcodes are essential for tracking and tracing products throughout the supply chain, and any errors can lead to significant issues such as product recalls or delays in distribution. The Barcode Verification System uses advanced imaging and scanning technologies to check the quality and readability of barcodes, ensuring they meet industry standards. Tag Presence Detection Systems are another vital part of this market. These systems are designed to verify the presence of specific tags or labels on pharmaceutical products. Tags can include RFID tags, holograms, or other security features that help in authenticating the product and preventing counterfeiting. The Tag Presence Detection System uses sensors and cameras to detect these tags and ensure they are correctly placed and intact. Other systems in the Global Inline Pharmaceutical Label Inspection Market include Optical Character Recognition (OCR) and Optical Character Verification (OCV) systems. These systems are used to read and verify the text on labels, ensuring that all information is correct and legible. OCR systems convert printed text into digital data, while OCV systems compare the printed text against a predefined standard to check for accuracy. These systems are crucial for ensuring that all information on the label, such as dosage instructions, expiration dates, and batch numbers, is correct. Additionally, there are systems designed to inspect the overall quality of the label, including its alignment, color, and print quality. These systems use high-resolution cameras and image processing software to detect any defects or inconsistencies in the label. By ensuring that labels are of high quality and free from errors, these systems help pharmaceutical companies maintain compliance with regulatory standards and ensure patient safety.
Large Enterprise, SME in the Global Inline Pharmaceutical Label Inspection Market:
The usage of the Global Inline Pharmaceutical Label Inspection Market varies significantly between large enterprises and small to medium-sized enterprises (SMEs). Large enterprises, with their extensive production lines and higher volumes of pharmaceutical products, require robust and scalable inspection systems. These companies often invest in advanced, high-speed inspection systems that can handle large quantities of products efficiently. For large enterprises, the primary focus is on ensuring compliance with stringent regulatory requirements and maintaining high standards of quality and safety. These companies often have dedicated quality control teams that work closely with the inspection systems to monitor and ensure the accuracy of labels. The use of inline pharmaceutical label inspection systems helps these companies avoid costly recalls, regulatory penalties, and damage to their reputation. On the other hand, SMEs may have different needs and constraints when it comes to label inspection. While they also need to comply with regulatory standards, their production volumes are typically lower, and they may have limited budgets for investing in advanced inspection systems. For SMEs, cost-effective and flexible inspection solutions are crucial. These companies may opt for modular systems that can be easily integrated into their existing production lines and scaled up as their business grows. The focus for SMEs is often on achieving a balance between cost and quality, ensuring that their products meet regulatory requirements without incurring excessive costs. Both large enterprises and SMEs benefit from the use of inline pharmaceutical label inspection systems in terms of improved efficiency, reduced errors, and enhanced product quality. By automating the inspection process, these systems help companies streamline their operations, reduce manual labor, and minimize the risk of human error. This is particularly important in the pharmaceutical industry, where even minor labeling errors can have serious consequences. Overall, the Global Inline Pharmaceutical Label Inspection Market plays a crucial role in helping both large enterprises and SMEs maintain compliance with regulatory standards, ensure patient safety, and protect their brand reputation.
Global Inline Pharmaceutical Label Inspection Market Outlook:
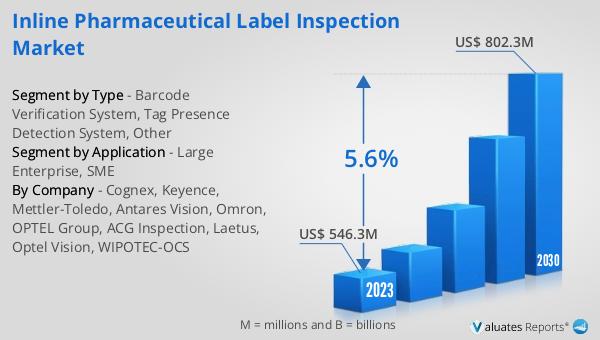
The global Inline Pharmaceutical Label Inspection market was valued at US$ 546.3 million in 2023 and is anticipated to reach US$ 802.3 million by 2030, witnessing a CAGR of 5.6% during the forecast period 2024-2030. This market outlook indicates a steady growth trajectory driven by the increasing need for accurate and reliable label inspection systems in the pharmaceutical industry. As regulatory requirements become more stringent and the focus on patient safety intensifies, the demand for advanced label inspection systems is expected to rise. The market's growth is also fueled by technological advancements in inspection systems, which offer improved accuracy, speed, and efficiency. Companies in the pharmaceutical industry are increasingly adopting these systems to ensure compliance with regulatory standards, reduce the risk of errors, and enhance overall product quality. The projected growth of the market reflects the critical importance of label inspection in maintaining the integrity and safety of pharmaceutical products. As the market continues to evolve, it is likely to see further innovations and advancements in inspection technologies, driving continued growth and adoption across the industry.
| Report Metric | Details |
| Report Name | Inline Pharmaceutical Label Inspection Market |
| Accounted market size in 2023 | US$ 546.3 million |
| Forecasted market size in 2030 | US$ 802.3 million |
| CAGR | 5.6% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Cognex, Keyence, Mettler-Toledo, Antares Vision, Omron, OPTEL Group, ACG Inspection, Laetus, Optel Vision, WIPOTEC-OCS |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
