What is Global Egg-Based Flu Vaccines Market?
The Global Egg-Based Flu Vaccines Market refers to the worldwide industry involved in the production and distribution of influenza vaccines that are developed using egg-based methods. These vaccines are created by injecting the flu virus into fertilized chicken eggs, allowing the virus to replicate. After a few days, the virus is harvested, inactivated, and purified to create the vaccine. This traditional method has been used for decades and remains a cornerstone in flu prevention strategies globally. The market encompasses various stakeholders, including pharmaceutical companies, healthcare providers, and regulatory bodies, all working together to ensure the availability and efficacy of these vaccines. The demand for egg-based flu vaccines is driven by the seasonal nature of influenza outbreaks, public health initiatives, and the need for effective immunization programs. As flu viruses continue to evolve, the market also focuses on research and development to improve vaccine formulations and production processes.
Trivalent Influenza Vaccine, Quadrivalent Influenza Vaccine in the Global Egg-Based Flu Vaccines Market:
The Global Egg-Based Flu Vaccines Market includes two primary types of vaccines: Trivalent Influenza Vaccine (TIV) and Quadrivalent Influenza Vaccine (QIV). Trivalent Influenza Vaccines are designed to protect against three different flu viruses: two influenza A viruses (H1N1 and H3N2) and one influenza B virus. These vaccines have been the standard for many years and are typically recommended for the general population, including children, adults, and the elderly. On the other hand, Quadrivalent Influenza Vaccines offer broader protection by including an additional influenza B virus strain. This means that QIVs protect against four flu viruses in total: two influenza A viruses and two influenza B viruses. The inclusion of the extra B strain aims to provide better coverage, especially in seasons where multiple B strains are circulating. Both TIV and QIV are produced using the same egg-based method, where the flu virus is injected into fertilized chicken eggs, allowed to replicate, and then harvested and purified. The choice between TIV and QIV often depends on factors such as age, health status, and specific recommendations from health authorities. While TIVs are generally sufficient for most people, QIVs are increasingly preferred due to their broader protection. The production process for both types of vaccines involves several steps, including virus propagation, inactivation, and purification, ensuring that the final product is safe and effective for public use. The market for these vaccines is influenced by various factors, including the prevalence of different flu strains, public health policies, and advancements in vaccine technology. As the flu virus continues to mutate, ongoing research and development are crucial to maintaining the efficacy of both TIV and QIV.
6 Months to 3 Years, > 3 Years in the Global Egg-Based Flu Vaccines Market:
The usage of Global Egg-Based Flu Vaccines Market can be categorized based on age groups, specifically for individuals aged 6 months to 3 years and those older than 3 years. For children aged 6 months to 3 years, flu vaccination is particularly important as their immune systems are still developing, making them more susceptible to severe flu complications. Pediatric formulations of egg-based flu vaccines are designed to be safe and effective for this age group, often administered in smaller doses compared to adults. Healthcare providers emphasize the importance of vaccinating young children to protect them from the flu and reduce the spread of the virus within communities. For individuals older than 3 years, including school-aged children, adults, and the elderly, flu vaccination remains a critical public health measure. The vaccines help prevent flu-related illnesses, hospitalizations, and deaths, especially among high-risk groups such as the elderly and those with underlying health conditions. In this age group, both Trivalent and Quadrivalent Influenza Vaccines are commonly used, with Quadrivalent vaccines gaining popularity due to their broader protection. Public health campaigns and vaccination programs aim to increase coverage rates and ensure that as many people as possible receive their annual flu shot. The Global Egg-Based Flu Vaccines Market plays a vital role in supporting these efforts by providing a reliable supply of vaccines tailored to different age groups and health needs.
Global Egg-Based Flu Vaccines Market Outlook:
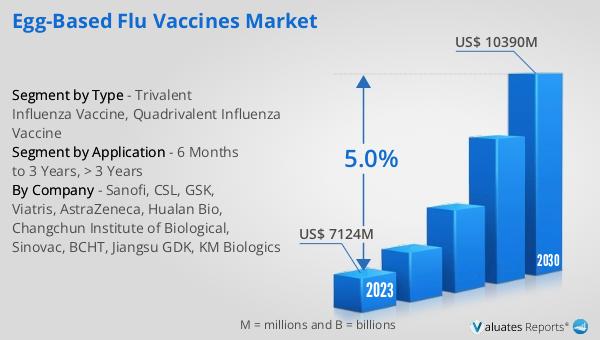
The global Egg-Based Flu Vaccines market was valued at US$ 7124 million in 2023 and is anticipated to reach US$ 10390 million by 2030, witnessing a CAGR of 5.0% during the forecast period 2024-2030. This growth reflects the increasing demand for effective flu vaccines, driven by the seasonal nature of influenza outbreaks and the ongoing efforts to improve public health through vaccination. The market's expansion is supported by advancements in vaccine production technology, research and development initiatives, and the collaboration of various stakeholders, including pharmaceutical companies, healthcare providers, and regulatory bodies. As the flu virus continues to evolve, the need for effective and reliable vaccines remains a top priority, ensuring that the Global Egg-Based Flu Vaccines Market will continue to play a crucial role in global health.
| Report Metric | Details |
| Report Name | Egg-Based Flu Vaccines Market |
| Accounted market size in 2023 | US$ 7124 million |
| Forecasted market size in 2030 | US$ 10390 million |
| CAGR | 5.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Sanofi, CSL, GSK, Viatris, AstraZeneca, Hualan Bio, Changchun Institute of Biological, Sinovac, BCHT, Jiangsu GDK, KM Biologics |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
