What is Global Palbociclib API Market?
The Global Palbociclib API Market refers to the worldwide market for the active pharmaceutical ingredient (API) used in the production of Palbociclib, a drug primarily used in the treatment of certain types of breast cancer. Palbociclib works by inhibiting specific proteins that are involved in the growth and division of cancer cells, thereby slowing down or stopping the progression of the disease. The market for Palbociclib API is driven by the increasing prevalence of breast cancer globally, advancements in pharmaceutical research, and the rising demand for effective cancer treatments. As more pharmaceutical companies invest in the development and production of Palbociclib, the market for its API is expected to grow. This growth is further supported by the expansion of healthcare infrastructure in emerging economies, which increases access to cancer treatments. Additionally, the market is influenced by regulatory approvals and patent expirations, which can affect the availability and pricing of Palbociclib. Overall, the Global Palbociclib API Market plays a crucial role in the pharmaceutical industry by providing the essential components needed to manufacture a drug that offers hope to many patients battling breast cancer.
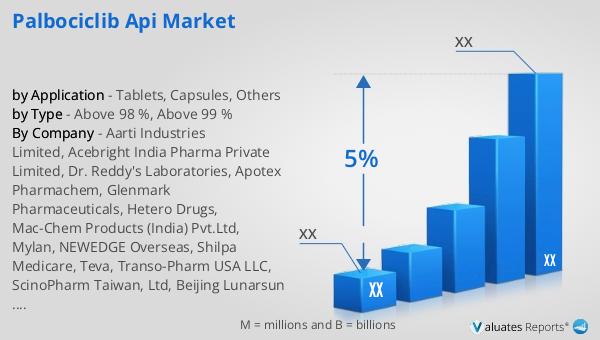
Above 98 %, Above 99 % in the Global Palbociclib API Market:
The Global Palbociclib API Market is segmented based on purity levels, with two primary categories being Above 98% and Above 99%. These purity levels are critical as they determine the quality and efficacy of the final pharmaceutical product. The Above 98% purity level indicates that the API contains a minimum of 98% of the active ingredient, with the remaining 2% comprising impurities or other substances. This level of purity is generally considered acceptable for many pharmaceutical applications, as it ensures that the drug is effective while maintaining safety standards. However, the Above 99% purity level represents a higher standard, with the API containing at least 99% of the active ingredient. This higher purity level is often preferred for more sensitive applications or where the highest efficacy is required. The choice between these purity levels depends on various factors, including the specific requirements of the drug formulation, regulatory standards, and cost considerations. Pharmaceutical companies may opt for the Above 99% purity level to ensure the highest quality and performance of their products, especially in competitive markets where differentiation is key. On the other hand, the Above 98% purity level may be chosen for cost-effectiveness, particularly in markets where price sensitivity is a significant factor. The production of high-purity APIs requires advanced manufacturing processes and stringent quality control measures to minimize impurities and ensure consistency. This involves sophisticated techniques such as crystallization, filtration, and chromatography, which help achieve the desired purity levels. The demand for high-purity Palbociclib API is also influenced by regulatory requirements, as health authorities in different regions may have specific guidelines regarding the acceptable purity levels for pharmaceutical products. Compliance with these regulations is essential for market access and can impact the competitiveness of API manufacturers. Furthermore, the choice of purity level can affect the overall cost of drug production, as higher purity APIs may require more complex and expensive manufacturing processes. This cost is often passed on to consumers, influencing the pricing strategy of pharmaceutical companies. In summary, the Global Palbociclib API Market is characterized by the availability of APIs with varying purity levels, each offering distinct advantages and challenges. The decision to use Above 98% or Above 99% purity APIs depends on a range of factors, including regulatory requirements, cost considerations, and the specific needs of the pharmaceutical product. As the market continues to evolve, manufacturers must balance these factors to meet the demands of healthcare providers and patients while maintaining competitiveness in the global market.
Tablets, Capsules, Others in the Global Palbociclib API Market:
The Global Palbociclib API Market finds its application in various pharmaceutical forms, including tablets, capsules, and other formulations. Tablets are one of the most common forms of medication delivery, offering convenience and ease of use for patients. In the context of Palbociclib, tablets are formulated to provide a precise dosage of the active ingredient, ensuring consistent therapeutic effects. The production of Palbociclib tablets involves the careful blending of the API with excipients, which are inactive substances that aid in the manufacturing process and enhance the stability and bioavailability of the drug. Tablets are often coated to improve their taste, protect the active ingredient from degradation, and control the release of the drug in the body. Capsules, on the other hand, offer an alternative form of drug delivery that can be advantageous for certain patients. Palbociclib capsules contain the API in a powdered or liquid form, enclosed within a gelatin or vegetarian shell. This form of delivery can be beneficial for patients who have difficulty swallowing tablets or require a formulation that allows for the rapid release of the active ingredient. Capsules can also be designed to provide controlled or sustained release of the drug, offering flexibility in dosing regimens. In addition to tablets and capsules, the Global Palbociclib API Market also encompasses other formulations, which may include liquid solutions, suspensions, or injectable forms. These alternative formulations can be tailored to meet specific patient needs or clinical requirements. For instance, liquid formulations may be preferred for pediatric or geriatric patients who have difficulty swallowing solid dosage forms. Injectable forms of Palbociclib may be used in clinical settings where rapid onset of action is required or when oral administration is not feasible. The choice of formulation is influenced by various factors, including the pharmacokinetic properties of Palbociclib, patient preferences, and the intended therapeutic use. Pharmaceutical companies must consider these factors when developing and marketing Palbociclib products to ensure they meet the diverse needs of patients and healthcare providers. The development of different formulations also requires adherence to stringent regulatory standards to ensure the safety, efficacy, and quality of the final product. In conclusion, the Global Palbociclib API Market supports a range of pharmaceutical formulations, each offering unique benefits and challenges. Tablets, capsules, and other forms of Palbociclib provide flexibility in drug delivery, catering to the diverse needs of patients and healthcare providers. As the market continues to grow, manufacturers must innovate and adapt to meet the evolving demands of the healthcare industry while ensuring the highest standards of quality and safety.
Global Palbociclib API Market Outlook:
The outlook for the Global Palbociclib API Market can be contextualized by examining the broader pharmaceutical and chemical drug markets. In 2022, the global pharmaceutical market was valued at approximately 1,475 billion USD, with an anticipated compound annual growth rate (CAGR) of 5% over the next six years. This growth is indicative of the increasing demand for pharmaceutical products worldwide, driven by factors such as the rising prevalence of chronic diseases, advancements in drug development, and expanding healthcare access in emerging economies. In comparison, the chemical drug market, which includes APIs like Palbociclib, has shown a steady increase from 1,005 billion USD in 2018 to an estimated 1,094 billion USD in 2022. This growth reflects the ongoing importance of chemical drugs in the treatment of various medical conditions, despite the rise of biologics and other advanced therapies. The chemical drug market's expansion is supported by the continuous innovation in drug formulations and the development of new APIs that address unmet medical needs. The Global Palbociclib API Market, as a part of this broader chemical drug market, benefits from these trends, as the demand for effective cancer treatments remains high. The market's growth is further bolstered by the increasing focus on personalized medicine and targeted therapies, which require high-quality APIs like Palbociclib. As pharmaceutical companies continue to invest in research and development, the Global Palbociclib API Market is poised to play a significant role in the future of cancer treatment, contributing to the overall growth of the pharmaceutical industry.
| Report Metric | Details |
| Report Name | Palbociclib API Market |
| CAGR | 5% |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Aarti Industries Limited, Acebright India Pharma Private Limited, Dr. Reddy's Laboratories, Apotex Pharmachem, Glenmark Pharmaceuticals, Hetero Drugs, Mac-Chem Products (India) Pvt.Ltd, Mylan, NEWEDGE Overseas, Shilpa Medicare, Teva, Transo-Pharm USA LLC, ScinoPharm Taiwan, Ltd, Beijing Lunarsun Pharmaceutical, Hangzhou Longshine Bio-Tech, Haoyuan Chemexpress Co.Ltd, Lunan Pharmaceutical, Shanghai Hope Chem |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |