What is Stirred Bioreactor System - Global Market?
Stirred bioreactor systems are a crucial component in the global market, primarily used for cultivating microorganisms or cells in a controlled environment. These systems are designed to provide optimal conditions for biological reactions, including temperature, pH, and oxygen levels, which are essential for the growth and productivity of cells. The global market for stirred bioreactor systems is driven by the increasing demand for biopharmaceuticals, vaccines, and other biologics. These systems are widely used in research and development, as well as in the production of pharmaceuticals and biofuels. The versatility of stirred bioreactors allows them to be used in various applications, from small-scale laboratory experiments to large-scale industrial production. As the biotechnology and pharmaceutical industries continue to grow, the demand for efficient and reliable bioreactor systems is expected to increase, making stirred bioreactors a vital part of the global market landscape. The advancements in technology and automation have further enhanced the efficiency and scalability of these systems, making them more accessible to a broader range of industries and applications.
Multi-use Bioreactor System, Single-use Bioreactor System in the Stirred Bioreactor System - Global Market:
The stirred bioreactor system market is divided into two main categories: multi-use and single-use bioreactor systems. Multi-use bioreactor systems are designed for repeated use, typically constructed from durable materials like stainless steel. These systems are ideal for large-scale production processes where the same bioreactor can be used multiple times, reducing the cost per batch. Multi-use systems are often equipped with advanced cleaning and sterilization capabilities, ensuring that they can be reused safely without contamination. They are particularly popular in industries where high-volume production is required, such as in the manufacturing of vaccines and other biopharmaceuticals. On the other hand, single-use bioreactor systems are designed for one-time use, often made from disposable materials like plastic. These systems are gaining popularity due to their flexibility and ease of use, especially in research and development settings where quick turnaround times are essential. Single-use systems eliminate the need for cleaning and sterilization, reducing the risk of cross-contamination and saving time and resources. They are particularly useful in clinical trials and small-scale production, where the focus is on speed and efficiency rather than large volumes. The choice between multi-use and single-use systems often depends on the specific needs of the application, including factors such as production scale, cost, and regulatory requirements. Both types of systems have their advantages and limitations, and the decision to use one over the other is often based on a careful analysis of these factors. As the global market for stirred bioreactor systems continues to evolve, manufacturers are constantly innovating to improve the performance and versatility of both multi-use and single-use systems. This includes the development of new materials and technologies that enhance the efficiency and scalability of these systems, making them more accessible to a wider range of industries and applications. The ongoing advancements in biotechnology and pharmaceuticals are expected to drive further growth in the stirred bioreactor system market, with both multi-use and single-use systems playing a crucial role in meeting the increasing demand for biopharmaceuticals and other biologics.
Biomanufacturing, Clinical Trials, Others in the Stirred Bioreactor System - Global Market:
Stirred bioreactor systems are extensively used in various areas, including biomanufacturing, clinical trials, and other applications. In biomanufacturing, these systems are essential for the production of biopharmaceuticals, vaccines, and other biologics. They provide a controlled environment for the cultivation of cells and microorganisms, ensuring optimal growth and productivity. The ability to scale up production from laboratory to industrial levels makes stirred bioreactors a vital tool in the biomanufacturing process. In clinical trials, stirred bioreactor systems are used to produce small batches of biologics for testing and evaluation. The flexibility and ease of use of single-use systems make them particularly suitable for this purpose, allowing researchers to quickly produce and test different formulations without the risk of cross-contamination. This is crucial in the fast-paced environment of clinical trials, where time is often of the essence. Beyond biomanufacturing and clinical trials, stirred bioreactor systems are also used in other applications, such as the production of biofuels and the development of new biotechnologies. Their versatility and scalability make them an attractive option for a wide range of industries, from pharmaceuticals to agriculture. The global market for stirred bioreactor systems is driven by the increasing demand for biopharmaceuticals and other biologics, as well as the ongoing advancements in biotechnology and pharmaceuticals. As these industries continue to grow, the demand for efficient and reliable bioreactor systems is expected to increase, making stirred bioreactors a vital part of the global market landscape. The advancements in technology and automation have further enhanced the efficiency and scalability of these systems, making them more accessible to a broader range of industries and applications.
Stirred Bioreactor System - Global Market Outlook:
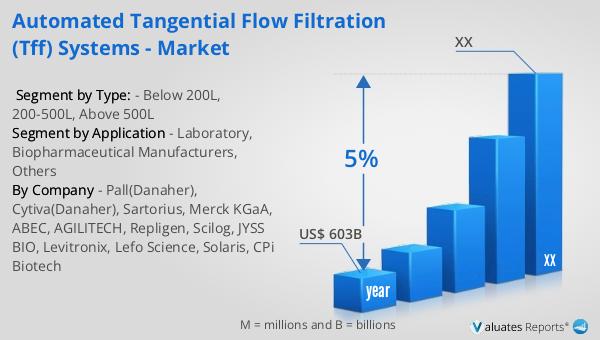
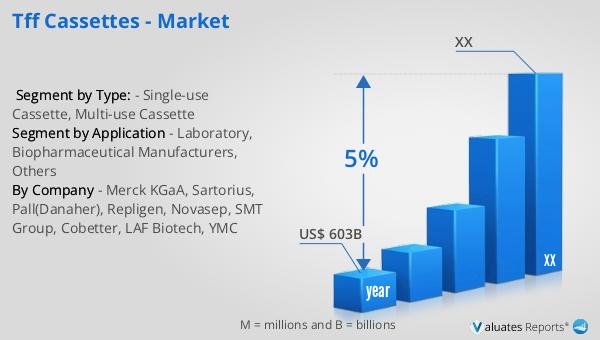
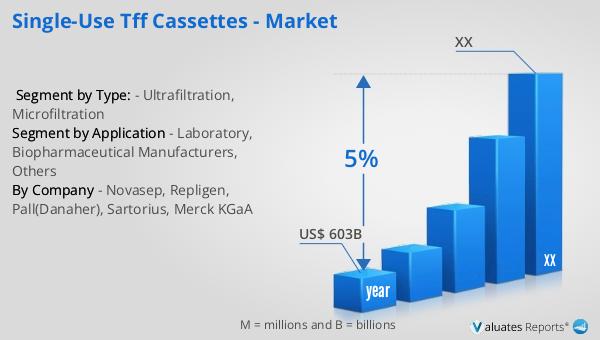
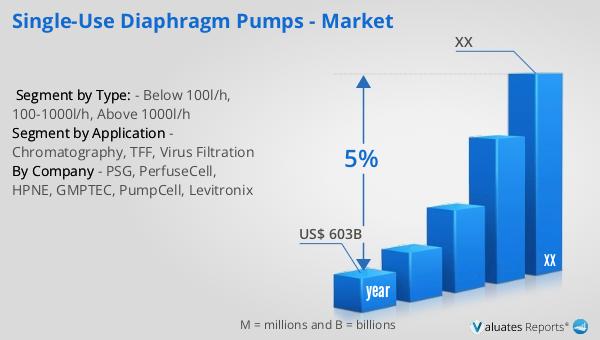
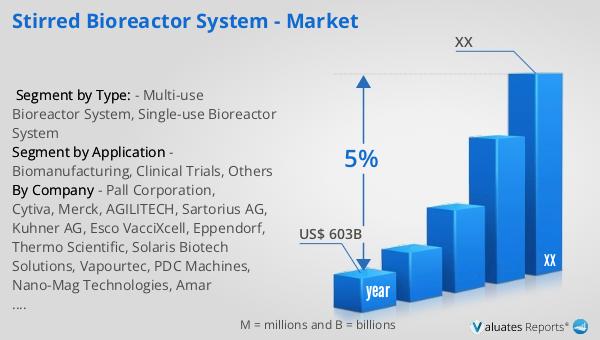
Based on our research, the global market for medical devices is projected to reach approximately $603 billion in 2023, with an anticipated growth rate of 5% annually over the next six years. This growth is driven by several factors, including the increasing demand for advanced medical technologies, the rising prevalence of chronic diseases, and the growing aging population worldwide. The medical device industry encompasses a wide range of products, from simple bandages to complex diagnostic equipment and surgical instruments. As healthcare systems around the world continue to evolve, there is a growing need for innovative medical devices that can improve patient outcomes and reduce healthcare costs. The development of new technologies, such as wearable devices and telemedicine solutions, is also contributing to the growth of the market. Additionally, the increasing focus on personalized medicine and the integration of artificial intelligence and machine learning in healthcare are expected to drive further innovation in the medical device industry. As the market continues to expand, companies are investing in research and development to create new products and improve existing ones, ensuring that they can meet the evolving needs of healthcare providers and patients. The global market for medical devices is expected to remain a key driver of growth in the healthcare industry, providing opportunities for companies to innovate and expand their product offerings.
| Report Metric | Details |
| Report Name | Stirred Bioreactor System - Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Pall Corporation, Cytiva, Merck, AGILITECH, Sartorius AG, Kuhner AG, Esco VacciXcell, Eppendorf, Thermo Scientific, Solaris Biotech Solutions, Vapourtec, PDC Machines, Nano-Mag Technologies, Amar Equipments, Weihai Global Chemical Machinery, Terralab Laboratory, Xiamen Ollital Technology |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |