What is Global Biopharma Storage Service Market?
The Global Biopharma Storage Service Market is a critical component of the pharmaceutical and biotechnology industries, providing essential storage solutions for a wide range of biological materials. These services are crucial for maintaining the integrity and efficacy of biopharmaceutical products, which include vaccines, biologics, and other temperature-sensitive materials. The market encompasses various storage solutions, each designed to meet specific temperature requirements necessary for preserving the stability and quality of biopharmaceuticals. As the demand for biopharmaceuticals continues to grow, driven by advancements in drug development and personalized medicine, the need for reliable storage solutions becomes increasingly important. Companies operating in this market offer a range of services, from ultra-low temperature storage to ambient temperature storage, ensuring that biopharmaceutical products are stored under optimal conditions. This market is characterized by technological advancements, stringent regulatory requirements, and a focus on sustainability and energy efficiency. As a result, the Global Biopharma Storage Service Market plays a vital role in supporting the pharmaceutical supply chain, enabling the safe and effective delivery of life-saving therapies to patients worldwide.
Ultra-Low Temperature Cryogenic Storage (-60℃ to -80℃), Frozen Storage (-25℃ to -15℃), Refrigerated Storage (2℃ to 8℃), Ambient/Controlled Room Temperature Storage (15℃ to 25℃), Gas-Phase Liquid Nitrogen Storage (Below -150℃) in the Global Biopharma Storage Service Market:
The Global Biopharma Storage Service Market offers a variety of storage solutions tailored to meet the specific needs of biopharmaceutical products. Ultra-Low Temperature Cryogenic Storage, ranging from -60℃ to -80℃, is essential for preserving the integrity of sensitive biological materials such as cell cultures, enzymes, and certain vaccines. This type of storage is crucial for long-term preservation, ensuring that these materials remain viable for future use. Frozen Storage, maintained between -25℃ to -15℃, is typically used for storing biological samples, reagents, and some pharmaceuticals that require a stable, cold environment to maintain their efficacy. Refrigerated Storage, with temperatures ranging from 2℃ to 8℃, is commonly used for vaccines, biologics, and other temperature-sensitive drugs that need to be kept cool but not frozen. This type of storage is vital for maintaining the potency and safety of these products throughout their shelf life. Ambient or Controlled Room Temperature Storage, maintained between 15℃ to 25℃, is suitable for products that are stable at room temperature but still require protection from extreme temperature fluctuations. This storage solution is often used for certain oral medications and over-the-counter products. Gas-Phase Liquid Nitrogen Storage, which operates at temperatures below -150℃, is used for the cryopreservation of cells, tissues, and other biological materials that require ultra-low temperatures to remain viable. This method is particularly important for preserving the genetic material and ensuring the long-term stability of cell lines used in research and therapy development. Each of these storage solutions plays a critical role in the biopharmaceutical supply chain, ensuring that products are stored under optimal conditions to maintain their quality and efficacy. As the biopharmaceutical industry continues to evolve, the demand for specialized storage solutions is expected to grow, driven by the increasing complexity of biopharmaceutical products and the need for stringent quality control measures.
Drug Development and Clinical Trials, Commercial Manufacturing and Supply Chain Management, Cell and Gene Therapy, Drug Traceability and Full Life Cycle Management, Others in the Global Biopharma Storage Service Market:
The Global Biopharma Storage Service Market is integral to various stages of the pharmaceutical and biotechnology industries, including drug development and clinical trials, commercial manufacturing and supply chain management, cell and gene therapy, drug traceability, and full life cycle management. In drug development and clinical trials, biopharma storage services ensure that experimental drugs and biological samples are stored under precise conditions to maintain their stability and efficacy. This is crucial for the success of clinical trials, as any deviation in storage conditions can compromise the integrity of the trial results. In commercial manufacturing and supply chain management, biopharma storage services play a vital role in ensuring that finished products are stored and transported under optimal conditions, preventing any degradation in quality. This is particularly important for temperature-sensitive products, such as vaccines and biologics, which require strict temperature control throughout the supply chain. In the field of cell and gene therapy, biopharma storage services are essential for preserving the viability of cells and genetic materials used in these advanced therapies. This includes the cryopreservation of cells at ultra-low temperatures to ensure their long-term stability and functionality. Drug traceability and full life cycle management are also supported by biopharma storage services, which provide the necessary infrastructure for tracking and monitoring the storage conditions of biopharmaceutical products throughout their lifecycle. This is important for ensuring compliance with regulatory requirements and maintaining the integrity of the supply chain. Additionally, biopharma storage services support other areas, such as research and development, by providing the necessary storage solutions for biological samples and reagents used in scientific studies. Overall, the Global Biopharma Storage Service Market is a critical component of the pharmaceutical and biotechnology industries, providing the necessary infrastructure to support the development, manufacturing, and distribution of life-saving therapies.
Global Biopharma Storage Service Market Outlook:
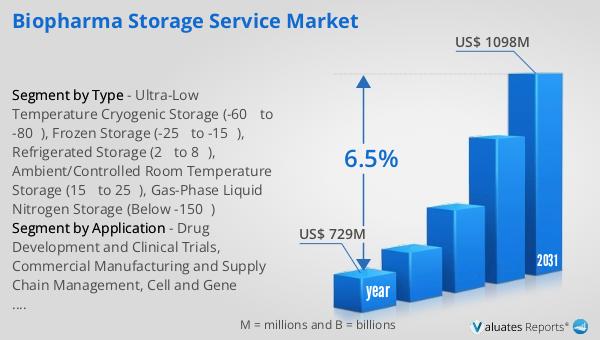
The global market for Biopharma Storage Service was valued at approximately $729 million in 2024, and it is anticipated to expand to a revised size of around $1,098 million by 2031. This growth represents a compound annual growth rate (CAGR) of 6.5% over the forecast period. This upward trajectory is indicative of the increasing demand for reliable and efficient storage solutions in the biopharmaceutical industry. As the industry continues to innovate and develop new therapies, the need for specialized storage solutions that can maintain the integrity and efficacy of these products becomes more critical. The market's growth is driven by several factors, including advancements in drug development, the rise of personalized medicine, and the increasing complexity of biopharmaceutical products. Additionally, stringent regulatory requirements and a focus on sustainability and energy efficiency are shaping the market landscape, prompting companies to invest in advanced storage technologies and infrastructure. As a result, the Global Biopharma Storage Service Market is poised to play a vital role in supporting the pharmaceutical supply chain, ensuring the safe and effective delivery of life-saving therapies to patients worldwide. This growth trajectory underscores the importance of biopharma storage services in the broader context of the pharmaceutical and biotechnology industries, highlighting their critical role in enabling the development and distribution of innovative therapies.
| Report Metric | Details |
| Report Name | Biopharma Storage Service Market |
| Accounted market size in year | US$ 729 million |
| Forecasted market size in 2031 | US$ 1098 million |
| CAGR | 6.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Supply Chain |
|
| Segment by Compliance |
|
| Segment by Application |
|
| By Region |
|
| By Company | Alcami, Azenta Life Sciences, Biopharma Technology LLC, Cencora, Donnegan Systems, ExtraVault, GBA Group, Patheon pharma services, PCI Services, Pharmaserv Logistics, SciSafe, Sentry BioPharma Services, Stirling Ultracold, Tobin Scientific |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
