What is Global Physical Therapy Center Market?
The Global Physical Therapy Center Market is a dynamic and essential segment of the healthcare industry, focusing on the provision of therapeutic services aimed at improving patients' physical functions and mobility. This market encompasses a wide range of services provided by trained physical therapists who work with individuals to alleviate pain, restore function, and prevent disability. Physical therapy centers are equipped with specialized equipment and facilities to cater to various patient needs, from rehabilitation after surgery to managing chronic conditions. The market is driven by factors such as an aging population, increasing prevalence of chronic diseases, and a growing awareness of the benefits of physical therapy. Additionally, advancements in technology and treatment methodologies have expanded the scope and effectiveness of physical therapy services. As healthcare systems worldwide emphasize preventive care and non-invasive treatment options, the demand for physical therapy centers continues to rise. These centers play a crucial role in enhancing the quality of life for patients by promoting physical activity, reducing pain, and improving overall health outcomes. The Global Physical Therapy Center Market is poised for growth as it adapts to changing healthcare needs and integrates innovative practices to meet the demands of diverse patient populations.
Hospitals, Clinic in the Global Physical Therapy Center Market:
Hospitals and clinics are integral components of the Global Physical Therapy Center Market, serving as primary venues for delivering physical therapy services. Hospitals, often equipped with comprehensive medical facilities, provide a wide range of physical therapy services as part of their inpatient and outpatient care. In a hospital setting, physical therapists work closely with other healthcare professionals to develop and implement individualized treatment plans for patients recovering from surgeries, injuries, or managing chronic conditions. These facilities are equipped with advanced technology and equipment, enabling therapists to offer specialized treatments such as hydrotherapy, electrotherapy, and manual therapy. Hospitals also serve as training grounds for physical therapy students and professionals, fostering an environment of continuous learning and innovation. Clinics, on the other hand, offer a more personalized and focused approach to physical therapy. They are often smaller in scale compared to hospitals but provide a wide array of services tailored to meet the specific needs of patients. Clinics may specialize in certain areas of physical therapy, such as sports rehabilitation, pediatric therapy, or geriatric care, allowing them to offer targeted and specialized treatments. The intimate setting of clinics often allows for more one-on-one interaction between therapists and patients, fostering a strong therapeutic relationship and enhancing treatment outcomes. Clinics are also more accessible to patients, often located in community settings, making it easier for individuals to seek care without the need for hospital admission. Both hospitals and clinics play a vital role in the Global Physical Therapy Center Market by providing essential services that cater to the diverse needs of patients. They contribute to the overall healthcare system by promoting recovery, enhancing mobility, and improving the quality of life for individuals across different age groups and health conditions. As the demand for physical therapy services continues to grow, hospitals and clinics are expanding their offerings and integrating innovative practices to meet the evolving needs of patients. This includes the adoption of telehealth services, which allow patients to receive therapy remotely, and the incorporation of advanced technologies such as virtual reality and robotics to enhance treatment effectiveness. The collaboration between hospitals and clinics also facilitates the sharing of knowledge and resources, leading to improved patient care and outcomes. In summary, hospitals and clinics are essential pillars of the Global Physical Therapy Center Market, providing comprehensive and specialized care that addresses the diverse needs of patients. Their role in delivering high-quality physical therapy services is crucial in promoting health and well-being, making them indispensable components of the healthcare system.
Orthopedic, Geriatric, Neurological, Cardiopulmonary, Pediatric in the Global Physical Therapy Center Market:
The Global Physical Therapy Center Market plays a significant role in various specialized areas, including orthopedic, geriatric, neurological, cardiopulmonary, and pediatric therapy. In orthopedic physical therapy, centers focus on treating musculoskeletal injuries and conditions such as fractures, sprains, and arthritis. Therapists work with patients to restore strength, flexibility, and function, often using exercises, manual therapy, and modalities like ultrasound or electrical stimulation. This area of therapy is crucial for individuals recovering from surgeries or injuries, helping them regain mobility and reduce pain. Geriatric physical therapy addresses the unique needs of the aging population, focusing on conditions such as osteoporosis, arthritis, and balance disorders. Physical therapy centers provide tailored programs to improve strength, balance, and coordination, reducing the risk of falls and enhancing the quality of life for older adults. Neurological physical therapy is another critical area, where therapists work with patients suffering from neurological disorders such as stroke, multiple sclerosis, or Parkinson's disease. The goal is to improve motor function, balance, and coordination through specialized exercises and techniques. Cardiopulmonary physical therapy focuses on patients with heart and lung conditions, aiming to improve cardiovascular endurance and respiratory function. Therapists design exercise programs to enhance the efficiency of the heart and lungs, helping patients manage symptoms and improve their overall health. Pediatric physical therapy centers on the developmental needs of children, addressing conditions such as cerebral palsy, developmental delays, and congenital disorders. Therapists use play-based activities and exercises to enhance motor skills, coordination, and strength, supporting children in reaching their developmental milestones. Each of these specialized areas within the Global Physical Therapy Center Market highlights the diverse applications and benefits of physical therapy in improving patient outcomes across different age groups and health conditions. By addressing specific needs and conditions, physical therapy centers contribute significantly to the overall health and well-being of individuals, promoting recovery, enhancing mobility, and improving the quality of life.
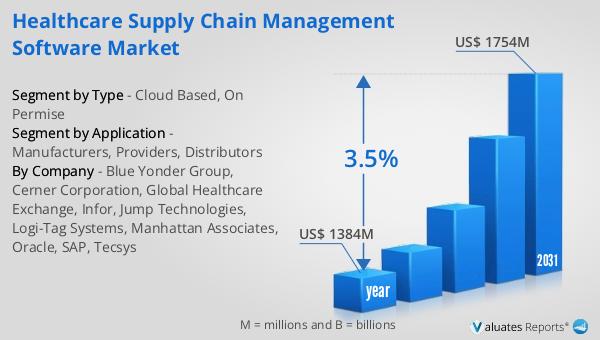
Global Physical Therapy Center Market Outlook:
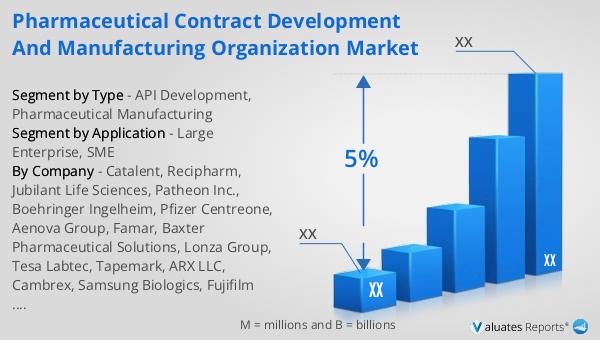
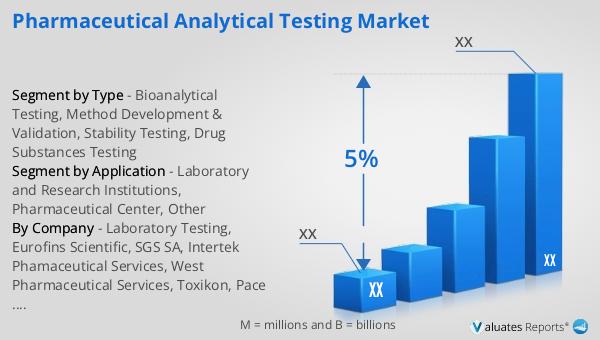
The global pharmaceutical market was valued at approximately 1,475 billion USD in 2022, reflecting a steady growth trajectory with a compound annual growth rate (CAGR) of 5% projected over the next six years. This growth is indicative of the increasing demand for pharmaceutical products and innovations in drug development and healthcare solutions. In comparison, the chemical drug market has also shown significant growth, with its value rising from 1,005 billion USD in 2018 to an estimated 1,094 billion USD in 2022. This increase underscores the expanding scope of chemical drugs within the broader pharmaceutical landscape, driven by advancements in research and development, as well as the introduction of new and effective treatments. The growth in both the pharmaceutical and chemical drug markets highlights the ongoing evolution and expansion of the healthcare industry, as it adapts to meet the needs of a growing global population and address emerging health challenges. These markets play a crucial role in providing essential medications and therapies that improve patient outcomes and enhance the quality of life. As the industry continues to innovate and expand, the global pharmaceutical and chemical drug markets are poised to remain key drivers of healthcare advancements and economic growth.
| Report Metric | Details |
| Report Name | Physical Therapy Center Market |
| CAGR | 5% |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Athletico Physical Therapy, ATI Physical Therapy, CORA Health Services, Pivot Physical Therapy, Professional Physical Therapy, PT Solutions, Select Medical, U.S. Physical Therapy, Shepherd Center, MossRehab, Rusk Rehabilitation Center, Craig Hospital, Spaulding Rehabilitation, Mayo Clinic, University of Washington Medical Center, TIRR Memorial Hermann, Rehabilitation Institute of Chicago |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |