What is In Vitro Diagnostics (IVD)- Global Market?
In Vitro Diagnostics (IVD) refers to a range of medical tests conducted on samples taken from the human body, such as blood, urine, or tissue, to detect diseases, conditions, or infections. These tests are performed outside the human body, hence the term "in vitro," which means "in glass" in Latin. The global market for IVD is vast and continuously evolving, driven by technological advancements, an aging population, and the increasing prevalence of chronic diseases. IVD plays a crucial role in healthcare by enabling early diagnosis, monitoring of disease progression, and guiding treatment decisions. The market encompasses a wide array of products, including reagents, instruments, and software, used in various settings such as hospitals, laboratories, and even at home. As healthcare systems worldwide strive for more personalized and efficient care, the demand for IVD solutions is expected to grow, making it a vital component of modern medical practice. The market's expansion is also fueled by the rising awareness of preventive healthcare and the need for rapid, accurate diagnostic tools.
Consumables, Equipment in the In Vitro Diagnostics (IVD)- Global Market:
In the realm of In Vitro Diagnostics (IVD), consumables and equipment form the backbone of the industry, each playing a pivotal role in the diagnostic process. Consumables, which include reagents, test kits, and other disposable items, are essential for conducting tests. They are the materials that interact directly with the samples to produce measurable results. Reagents, for instance, are chemical substances used to detect or quantify other substances in a sample. Test kits, on the other hand, are pre-packaged sets of reagents and other materials designed for specific tests, making them convenient and user-friendly. The demand for consumables is high due to their single-use nature, which ensures accuracy and prevents contamination. This segment dominates the IVD market, reflecting its critical importance in the diagnostic process. On the other hand, equipment refers to the instruments and machines used to perform IVD tests. These range from simple devices like glucometers to complex automated systems used in large laboratories. Equipment is crucial for processing samples, analyzing results, and ensuring precision and reliability. Technological advancements have led to the development of sophisticated equipment that can perform multiple tests simultaneously, increasing efficiency and throughput. The integration of artificial intelligence and machine learning in IVD equipment is also enhancing diagnostic capabilities, allowing for more accurate and faster results. Both consumables and equipment are indispensable in the IVD market, working in tandem to provide comprehensive diagnostic solutions. The synergy between these components ensures that healthcare providers can deliver timely and accurate diagnoses, ultimately improving patient outcomes. As the IVD market continues to grow, innovations in consumables and equipment will likely drive further advancements in diagnostic testing, making healthcare more accessible and effective.
Hospital, Household in the In Vitro Diagnostics (IVD)- Global Market:
In Vitro Diagnostics (IVD) is extensively used in hospitals and households, each setting presenting unique applications and benefits. In hospitals, IVD plays a critical role in patient care, from diagnosis to treatment monitoring. Hospitals rely on a wide range of IVD tests to diagnose diseases, assess health conditions, and monitor treatment efficacy. For instance, blood tests can detect infections, measure glucose levels, or assess organ function, providing crucial information for medical decision-making. IVD tests in hospitals are often conducted in centralized laboratories equipped with advanced instruments capable of processing large volumes of samples quickly and accurately. This capability is vital for managing patient flow and ensuring timely diagnoses, which can significantly impact treatment outcomes. Moreover, the integration of IVD with hospital information systems allows for seamless data sharing and analysis, enhancing the overall efficiency of healthcare delivery. In households, IVD offers the convenience of at-home testing, empowering individuals to monitor their health proactively. Home-based IVD tests, such as pregnancy tests, glucose monitors, and cholesterol kits, provide users with immediate results, enabling them to make informed health decisions without visiting a healthcare facility. This accessibility is particularly beneficial for individuals with chronic conditions who require regular monitoring. The rise of telemedicine and digital health platforms has further expanded the use of IVD in households, allowing users to share test results with healthcare providers remotely and receive timely guidance. The convenience and accessibility of home-based IVD tests contribute to increased health awareness and preventive care, ultimately reducing the burden on healthcare systems. Both hospital and household applications of IVD underscore its importance in modern healthcare, offering solutions that cater to diverse needs and settings. As technology continues to advance, the scope and capabilities of IVD are expected to expand, further enhancing its role in promoting health and well-being.
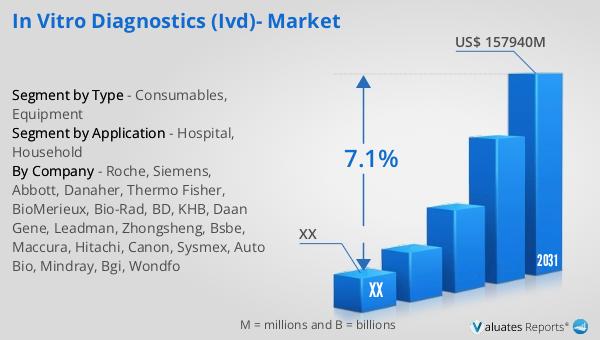
In Vitro Diagnostics (IVD)- Global Market Outlook:
The global market for In Vitro Diagnostics (IVD) is poised for significant growth in the coming years. In 2024, the market was valued at approximately US$ 98,430 million, and projections indicate that it will reach an adjusted size of US$ 157,940 million by 2031. This growth trajectory reflects a compound annual growth rate (CAGR) of 7.1% during the forecast period from 2025 to 2031. A notable aspect of the market is the concentration of market share among the leading manufacturers, with the top four companies collectively holding around 42% of the global market share. This concentration underscores the competitive nature of the industry and the importance of innovation and strategic partnerships in maintaining market leadership. In terms of product types, consumables represent the largest segment, accounting for a substantial 87% share of the market. This dominance is attributed to the essential role consumables play in the diagnostic process, as they are critical for conducting tests and ensuring accurate results. The high demand for consumables is driven by their single-use nature, which minimizes the risk of contamination and ensures the reliability of test outcomes. As the IVD market continues to evolve, the focus on consumables is expected to remain strong, supported by ongoing advancements in diagnostic technologies and the growing emphasis on personalized healthcare.
| Report Metric | Details |
| Report Name | In Vitro Diagnostics (IVD)- Market |
| Forecasted market size in 2031 | US$ 157940 million |
| CAGR | 7.1% |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Roche, Siemens, Abbott, Danaher, Thermo Fisher, BioMerieux, Bio-Rad, BD, KHB, Daan Gene, Leadman, Zhongsheng, Bsbe, Maccura, Hitachi, Canon, Sysmex, Auto Bio, Mindray, Bgi, Wondfo |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |