What is Global Recombinant Human Coagulation VIIa Market?
The Global Recombinant Human Coagulation VIIa Market is a specialized segment within the pharmaceutical industry that focuses on the production and distribution of a specific type of medication used to treat bleeding disorders. Recombinant Human Coagulation Factor VIIa is a protein that plays a crucial role in the blood coagulation process, helping to control bleeding in individuals who have certain medical conditions that affect their ability to form blood clots. This market is driven by the increasing prevalence of bleeding disorders, advancements in biotechnology, and the growing demand for effective and safe treatment options. The market encompasses various products, including prefilled syringes and vials, which are designed to deliver precise doses of the medication to patients. The development and commercialization of these products involve extensive research and regulatory approvals to ensure their safety and efficacy. As the global population continues to age and the incidence of bleeding disorders rises, the demand for Recombinant Human Coagulation VIIa is expected to grow, making this market an important area of focus for pharmaceutical companies and healthcare providers worldwide.
Prefilled Syringe, Vial in the Global Recombinant Human Coagulation VIIa Market:
Prefilled syringes and vials are two primary forms of packaging and delivery systems used in the Global Recombinant Human Coagulation VIIa Market. Prefilled syringes are designed to provide a convenient and ready-to-use solution for administering the medication. They are preloaded with a specific dose of Recombinant Human Coagulation VIIa, which eliminates the need for manual preparation and reduces the risk of dosing errors. This form of packaging is particularly beneficial in emergency situations where time is of the essence, as it allows for quick and accurate administration of the medication. Prefilled syringes are also designed to minimize the risk of contamination, as they are sealed and sterile until the point of use. This is an important consideration in the treatment of bleeding disorders, where maintaining a sterile environment is crucial to prevent infections. On the other hand, vials are a more traditional form of packaging that requires the medication to be drawn into a syringe before administration. While this method may require more preparation time compared to prefilled syringes, it offers greater flexibility in terms of dosing. Vials are typically used in hospital settings where healthcare professionals have the expertise and equipment to prepare the medication accurately. They are also more cost-effective for larger doses, making them a preferred choice in certain clinical scenarios. Both prefilled syringes and vials have their own advantages and limitations, and the choice between the two often depends on the specific needs of the patient and the healthcare setting. The development of these delivery systems involves rigorous testing and quality control to ensure that they meet the highest standards of safety and efficacy. Manufacturers must adhere to strict regulatory guidelines to ensure that their products are safe for use and provide the intended therapeutic benefits. As the demand for Recombinant Human Coagulation VIIa continues to grow, there is ongoing research and innovation in the development of new and improved delivery systems. This includes the exploration of novel materials and technologies that can enhance the stability and shelf life of the medication, as well as improve the ease of use for patients and healthcare providers. The Global Recombinant Human Coagulation VIIa Market is a dynamic and evolving field, with continuous advancements in packaging and delivery systems playing a key role in the overall growth and development of the market.
Congenital Hemophilia, Acquired Hemophilia, Others in the Global Recombinant Human Coagulation VIIa Market:
The usage of Global Recombinant Human Coagulation VIIa Market products is primarily focused on the treatment of bleeding disorders such as Congenital Hemophilia, Acquired Hemophilia, and other related conditions. Congenital Hemophilia is a genetic disorder characterized by the deficiency or absence of specific clotting factors, leading to prolonged bleeding episodes. Recombinant Human Coagulation VIIa is used as a treatment option for patients with Congenital Hemophilia who have developed inhibitors against standard clotting factor replacement therapies. It works by bypassing the missing or deficient clotting factors and promoting the formation of a stable blood clot, thereby controlling bleeding. This is particularly important in managing bleeding episodes that occur spontaneously or as a result of injury or surgery. Acquired Hemophilia, on the other hand, is a rare autoimmune disorder where the body's immune system mistakenly attacks its own clotting factors, leading to severe bleeding. Recombinant Human Coagulation VIIa is used as a first-line treatment in these cases, as it can effectively control bleeding by providing an alternative pathway for clot formation. The use of this medication is crucial in preventing life-threatening bleeding complications and improving the quality of life for patients with Acquired Hemophilia. In addition to these specific conditions, Recombinant Human Coagulation VIIa is also used in other clinical scenarios where there is a need for rapid and effective control of bleeding. This includes situations such as trauma, surgery, and other medical procedures where there is a high risk of bleeding. The versatility and efficacy of Recombinant Human Coagulation VIIa make it a valuable tool in the management of various bleeding disorders. The administration of this medication requires careful monitoring and dose adjustment to ensure optimal therapeutic outcomes. Healthcare providers must consider factors such as the severity of the bleeding, the patient's overall health status, and any potential interactions with other medications. The use of Recombinant Human Coagulation VIIa is supported by extensive clinical research and guidelines from leading medical organizations, which provide evidence-based recommendations for its use in different clinical settings. As the understanding of bleeding disorders continues to evolve, there is ongoing research into new indications and potential applications for Recombinant Human Coagulation VIIa. This includes exploring its use in combination with other therapies and investigating its potential benefits in other medical conditions. The Global Recombinant Human Coagulation VIIa Market plays a critical role in providing effective treatment options for patients with bleeding disorders, and its continued development and innovation are essential for advancing patient care and improving outcomes.
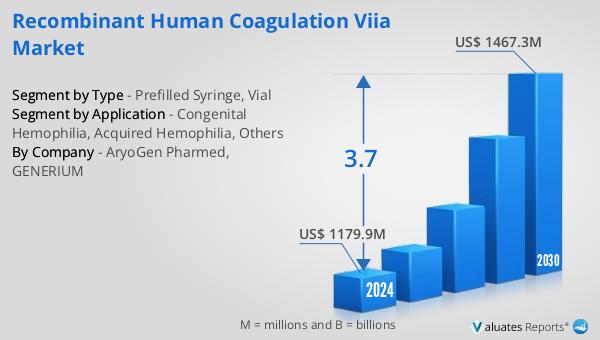
Global Recombinant Human Coagulation VIIa Market Outlook:
The global market for Recombinant Human Coagulation VIIa was valued at $1,251 million in 2024 and is anticipated to expand to a revised size of $1,608 million by 2031, reflecting a compound annual growth rate (CAGR) of 3.7% over the forecast period. The market is dominated by three major manufacturers: Novo Nordisk, LFB SA, HEMA Biologics, and GENERIUM, which collectively hold a market share exceeding 99%. Among these, Novo Nordisk stands out as the largest manufacturer, commanding a market share of over 95%. North America emerges as the most significant consumer market for Recombinant Human Coagulation VIIa, accounting for approximately 45% of the global market share. This dominance is attributed to the region's advanced healthcare infrastructure, high prevalence of bleeding disorders, and strong focus on research and development. The market's growth is driven by factors such as increasing awareness of bleeding disorders, advancements in biotechnology, and the rising demand for effective treatment options. As the market continues to evolve, manufacturers are focusing on innovation and strategic partnerships to enhance their product offerings and expand their global presence. The Global Recombinant Human Coagulation VIIa Market is poised for steady growth, with ongoing research and development efforts aimed at improving the safety, efficacy, and accessibility of these life-saving treatments.
| Report Metric | Details |
| Report Name | Recombinant Human Coagulation VIIa Market |
| Accounted market size in year | US$ 1251 million |
| Forecasted market size in 2031 | US$ 1608 million |
| CAGR | 3.7% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Novo Nordisk, LFB SA HEMA Biologics, AryoGen Pharmed, GENERIUM |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |