What is Global GMP Cell Banking Market?
The Global GMP Cell Banking Market is a specialized sector within the biotechnology and pharmaceutical industries that focuses on the creation, storage, and maintenance of cell banks under Good Manufacturing Practice (GMP) conditions. These cell banks are crucial for the production of biopharmaceuticals, including vaccines, monoclonal antibodies, and other therapeutic proteins. GMP cell banking ensures that the cells used in production are consistent, safe, and of high quality, which is essential for regulatory compliance and the efficacy of the final product. The market is driven by the increasing demand for biopharmaceuticals, advancements in cell culture technologies, and the need for standardized and reliable cell lines. Companies operating in this market provide services such as cell line development, characterization, and storage, adhering to stringent regulatory guidelines. The market is expected to grow significantly as more pharmaceutical companies and research institutions recognize the importance of GMP cell banking in ensuring the quality and safety of biopharmaceutical products. This growth is further supported by the rising prevalence of chronic diseases and the ongoing development of personalized medicine, which relies heavily on biopharmaceuticals produced from GMP-compliant cell banks.
Mammalian Cell, Microbial Cell, Insect Cell, Others in the Global GMP Cell Banking Market:
In the Global GMP Cell Banking Market, various types of cells are utilized, each serving distinct purposes in biopharmaceutical production. Mammalian cells are the most commonly used due to their ability to produce complex proteins with post-translational modifications similar to those in humans. These cells, such as Chinese Hamster Ovary (CHO) cells and Human Embryonic Kidney (HEK) cells, are preferred for producing therapeutic proteins and monoclonal antibodies. Their use is driven by the need for high-quality, human-like proteins that are safe and effective for therapeutic use. The process of developing mammalian cell banks involves rigorous testing and validation to ensure the cells' stability, productivity, and compliance with GMP standards. Microbial cells, including bacteria like Escherichia coli and yeast such as Saccharomyces cerevisiae, are also widely used in the GMP Cell Banking Market. These cells are favored for their rapid growth, ease of genetic manipulation, and cost-effectiveness. They are primarily used for producing simpler proteins and enzymes that do not require complex post-translational modifications. The development of microbial cell banks involves optimizing growth conditions and genetic stability to ensure consistent production yields. Insect cells, such as those derived from the Spodoptera frugiperda (Sf9) and Trichoplusia ni (Tni) cell lines, are another important component of the GMP Cell Banking Market. These cells are used in the baculovirus expression vector system (BEVS) to produce recombinant proteins and vaccines. Insect cells offer advantages such as high protein expression levels and the ability to perform some post-translational modifications. The establishment of insect cell banks requires careful selection and characterization of cell lines to ensure their suitability for large-scale production. Other cell types, including plant cells and stem cells, are also gaining attention in the GMP Cell Banking Market. Plant cells are explored for producing biopharmaceuticals due to their scalability and low risk of contamination with human pathogens. Stem cells, on the other hand, hold promise for regenerative medicine and cell therapy applications. The development of cell banks for these cell types involves unique challenges, such as optimizing culture conditions and ensuring genetic stability. Overall, the Global GMP Cell Banking Market encompasses a diverse range of cell types, each with specific advantages and applications. The choice of cell type depends on factors such as the complexity of the protein being produced, the required post-translational modifications, and the intended therapeutic application. As the demand for biopharmaceuticals continues to grow, the development and maintenance of GMP-compliant cell banks will remain a critical aspect of ensuring the quality and safety of these products.
Biopharmaceutical Companies, Contract Manufacturing Organizations in the Global GMP Cell Banking Market:
The Global GMP Cell Banking Market plays a crucial role in the operations of biopharmaceutical companies and contract manufacturing organizations (CMOs). Biopharmaceutical companies rely on GMP cell banks to ensure the consistent production of high-quality therapeutic proteins and vaccines. These companies invest in the development and maintenance of cell banks to meet regulatory requirements and to ensure the safety and efficacy of their products. GMP cell banks provide a reliable source of well-characterized cell lines that are essential for large-scale production processes. The use of GMP-compliant cell banks helps biopharmaceutical companies streamline their production processes, reduce the risk of contamination, and ensure product consistency. This is particularly important in the production of monoclonal antibodies and other complex biologics, where even minor variations in cell lines can impact the final product's quality and efficacy. Contract Manufacturing Organizations (CMOs) also benefit significantly from the Global GMP Cell Banking Market. CMOs provide manufacturing services to biopharmaceutical companies, and having access to GMP-compliant cell banks allows them to offer high-quality production services. CMOs often maintain their own cell banks or collaborate with specialized cell banking service providers to ensure they can meet their clients' production needs. The availability of GMP cell banks enables CMOs to offer flexible and scalable manufacturing solutions, catering to the diverse requirements of their clients. This is particularly valuable for smaller biopharmaceutical companies that may not have the resources to develop and maintain their own cell banks. By leveraging GMP cell banks, CMOs can help these companies bring their products to market more efficiently and cost-effectively. In summary, the Global GMP Cell Banking Market is integral to the success of both biopharmaceutical companies and CMOs. It provides the foundation for producing safe, effective, and high-quality biopharmaceutical products, ensuring compliance with regulatory standards and meeting the growing demand for biologics. As the biopharmaceutical industry continues to expand, the importance of GMP cell banking in supporting production processes and ensuring product quality will only increase.
Global GMP Cell Banking Market Outlook:
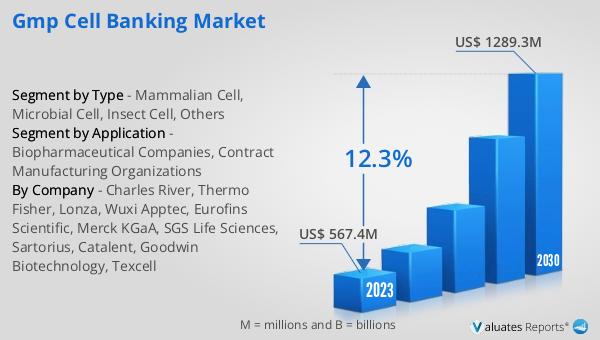
The global market for GMP Cell Banking was valued at approximately $715 million in 2024, with projections indicating a significant growth trajectory, reaching an estimated $1,589 million by 2031. This growth is expected to occur at a compound annual growth rate (CAGR) of 12.3% over the forecast period. The market is characterized by a high concentration of key players, with the top five manufacturers collectively holding a market share exceeding 55%. Among the various product segments, mammalian cells emerge as the largest, commanding a share of over 63%. This dominance is attributed to the widespread use of mammalian cells in the production of complex therapeutic proteins and monoclonal antibodies, which require post-translational modifications similar to those in human cells. The preference for mammalian cells is driven by their ability to produce high-quality, human-like proteins that are safe and effective for therapeutic use. The market's growth is further supported by the increasing demand for biopharmaceuticals, advancements in cell culture technologies, and the need for standardized and reliable cell lines. As the biopharmaceutical industry continues to expand, the Global GMP Cell Banking Market is poised to play a critical role in ensuring the quality and safety of biopharmaceutical products.
| Report Metric | Details |
| Report Name | GMP Cell Banking Market |
| Accounted market size in year | US$ 715 million |
| Forecasted market size in 2031 | US$ 1589 million |
| CAGR | 12.3% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Charles River, Thermo Fisher, Lonza, Wuxi Apptec, Eurofins Scientific, Merck KGaA, SGS Life Sciences, Sartorius, Catalent, Goodwin Biotechnology, Texcell, Precision for Medicine, Kryosphere, ProBi |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |