What is Global Human Coagulation Factor XIII Market?
The Global Human Coagulation Factor XIII Market is a specialized segment within the broader pharmaceutical and healthcare industry, focusing on the production and distribution of Factor XIII, a crucial protein involved in blood coagulation. Factor XIII plays a vital role in stabilizing blood clots, which is essential for wound healing and preventing excessive bleeding. This market encompasses various products and formulations designed to supplement or replace Factor XIII in individuals who have deficiencies due to genetic disorders, medical conditions, or surgical procedures. The demand for Human Coagulation Factor XIII is driven by its critical application in treating bleeding disorders such as congenital Factor XIII deficiency, which, although rare, can lead to severe bleeding complications if not managed properly. Additionally, advancements in biotechnology and increased awareness about bleeding disorders have contributed to the growth of this market. The market is characterized by ongoing research and development efforts aimed at improving the efficacy and safety of Factor XIII products, as well as expanding their applications in various medical fields. As healthcare systems worldwide continue to prioritize patient safety and effective treatment options, the Global Human Coagulation Factor XIII Market is expected to witness sustained growth and innovation.
Lyophilized Powder, Liquid in the Global Human Coagulation Factor XIII Market:
In the Global Human Coagulation Factor XIII Market, two primary formulations are prevalent: lyophilized powder and liquid. Lyophilized powder, also known as freeze-dried powder, is a form of Factor XIII that has been dehydrated to enhance its stability and shelf life. This formulation is particularly advantageous for storage and transportation, as it does not require refrigeration and can be reconstituted with a diluent before administration. The lyophilization process involves freezing the Factor XIII solution and then removing the water content through sublimation, resulting in a dry powder that retains the protein's biological activity. This method is widely used in the pharmaceutical industry to preserve the integrity of sensitive biological products, making it a preferred choice for many healthcare providers and patients. On the other hand, the liquid formulation of Factor XIII is ready-to-use and does not require reconstitution, offering convenience and ease of administration. This form is typically stored under refrigerated conditions to maintain its stability and efficacy. The choice between lyophilized powder and liquid formulations often depends on the specific needs of the healthcare setting, patient preferences, and logistical considerations. For instance, in emergency situations or surgical settings where time is of the essence, the liquid formulation may be preferred due to its immediate availability. Conversely, in regions with limited access to refrigeration or where long-term storage is necessary, the lyophilized powder may be more suitable. Both formulations are designed to deliver the therapeutic benefits of Factor XIII effectively, ensuring that patients receive the necessary support for blood coagulation and wound healing. The development and refinement of these formulations are driven by ongoing research and technological advancements in the field of biopharmaceuticals. Manufacturers are continually exploring ways to enhance the stability, bioavailability, and safety of Factor XIII products, aiming to improve patient outcomes and expand the market's reach. As the Global Human Coagulation Factor XIII Market evolves, the interplay between lyophilized powder and liquid formulations will continue to shape the landscape of treatment options available to patients with bleeding disorders.
Surgery, Disease Treatment, Medical Research, Others in the Global Human Coagulation Factor XIII Market:
The Global Human Coagulation Factor XIII Market finds its application in several critical areas, including surgery, disease treatment, medical research, and other specialized fields. In the context of surgery, Factor XIII is used to enhance hemostasis, the process that stops bleeding, by stabilizing blood clots and promoting wound healing. This is particularly important in complex surgical procedures where excessive bleeding can pose significant risks to patient safety. Surgeons often rely on Factor XIII supplementation to ensure that patients have adequate clotting ability during and after surgery, reducing the likelihood of complications and improving recovery outcomes. In disease treatment, Factor XIII is primarily used to manage congenital Factor XIII deficiency, a rare genetic disorder that impairs the body's ability to form stable blood clots. Patients with this condition are at risk of spontaneous bleeding episodes, which can be life-threatening if not properly managed. Regular administration of Factor XIII helps to prevent these episodes and maintain normal clotting function, allowing patients to lead healthier lives. Additionally, Factor XIII is being explored for its potential therapeutic benefits in other bleeding disorders and conditions that affect coagulation, expanding its role in disease management. In the realm of medical research, Factor XIII is a subject of ongoing investigation as scientists seek to understand its broader implications in coagulation and wound healing. Research efforts are focused on elucidating the molecular mechanisms of Factor XIII activation and its interactions with other components of the coagulation cascade. These studies aim to uncover new therapeutic targets and improve the efficacy of existing treatments, contributing to the advancement of medical knowledge and innovation in the field. Beyond these primary applications, Factor XIII is also utilized in other specialized areas, such as trauma care and obstetrics, where effective blood clotting is crucial for patient outcomes. In trauma care, rapid administration of Factor XIII can be lifesaving for patients experiencing severe bleeding due to injury. In obstetrics, it may be used to manage bleeding complications during childbirth, ensuring the safety of both mother and child. The versatility of Factor XIII in addressing various medical needs underscores its importance in the healthcare landscape and highlights the ongoing demand for effective coagulation therapies.
Global Human Coagulation Factor XIII Market Outlook:
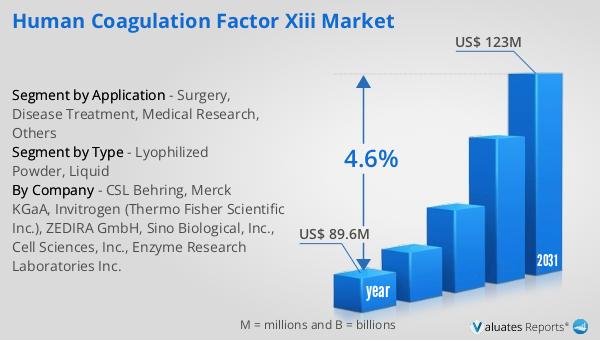
The outlook for the Global Human Coagulation Factor XIII Market indicates a promising trajectory, with the market valued at approximately US$ 89.6 million in 2024. It is anticipated to grow steadily, reaching an estimated size of US$ 123 million by 2031. This growth is expected to occur at a compound annual growth rate (CAGR) of 4.6% over the forecast period. The market's expansion can be attributed to several factors, including increasing awareness of bleeding disorders and the critical role of Factor XIII in managing these conditions. As healthcare systems worldwide continue to prioritize patient safety and effective treatment options, the demand for Factor XIII products is likely to rise. Additionally, advancements in biotechnology and pharmaceutical research are expected to drive innovation in the development of new and improved Factor XIII formulations, further fueling market growth. The steady increase in market size reflects the growing recognition of Factor XIII's importance in various medical applications, from surgery and disease treatment to medical research and beyond. As the market evolves, stakeholders, including manufacturers, healthcare providers, and researchers, will continue to collaborate to enhance the availability and accessibility of Factor XIII products, ensuring that patients receive the best possible care. The projected growth of the Global Human Coagulation Factor XIII Market underscores the ongoing commitment to advancing healthcare solutions and improving patient outcomes in the field of coagulation therapy.
| Report Metric | Details |
| Report Name | Human Coagulation Factor XIII Market |
| Accounted market size in year | US$ 89.6 million |
| Forecasted market size in 2031 | US$ 123 million |
| CAGR | 4.6% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | CSL Behring, Merck KGaA, Invitrogen (Thermo Fisher Scientific Inc.), ZEDIRA GmbH, Sino Biological, Inc., Cell Sciences, Inc., Enzyme Research Laboratories Inc. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
