What is Global Levonorgestrel Implant Market?
The global Levonorgestrel Implant market is a specialized segment within the broader pharmaceutical industry, focusing on the production and distribution of Levonorgestrel implants. These implants are a form of long-acting reversible contraception (LARC) that release the hormone Levonorgestrel to prevent pregnancy. They are typically inserted under the skin of the upper arm and can provide effective contraception for several years. The market for these implants is driven by the increasing demand for reliable and long-term contraceptive methods, particularly in regions with high birth rates and limited access to healthcare. Additionally, the convenience and efficacy of Levonorgestrel implants make them a popular choice among women seeking to avoid the daily hassle of taking oral contraceptives. The market is also influenced by factors such as government initiatives promoting family planning, advancements in medical technology, and growing awareness about reproductive health. Overall, the global Levonorgestrel Implant market plays a crucial role in addressing the contraceptive needs of women worldwide, contributing to better family planning and improved quality of life.
2 Root Type, 6 Root Type in the Global Levonorgestrel Implant Market:
The Global Levonorgestrel Implant Market can be categorized based on the type of implant, primarily focusing on 2 Root Type and 6 Root Type implants. The 2 Root Type Levonorgestrel implants are designed with two rods that are inserted under the skin. These rods release a steady dose of Levonorgestrel over a period of time, typically up to five years. The dual-rod system ensures a consistent release of the hormone, providing reliable contraception. This type is often preferred for its simplicity and ease of insertion and removal. On the other hand, the 6 Root Type Levonorgestrel implants consist of six smaller rods or capsules that are also inserted under the skin. These multiple rods work together to release the hormone over a similar duration, offering an alternative for those who may have specific medical or personal preferences. The choice between 2 Root Type and 6 Root Type implants can depend on various factors, including the patient's medical history, the healthcare provider's recommendation, and individual comfort levels. Both types are highly effective in preventing pregnancy and are part of the broader effort to provide diverse contraceptive options to meet the varying needs of women globally. The market for these implants is supported by ongoing research and development, aiming to improve the efficacy, safety, and user experience of these contraceptive methods. As healthcare providers and patients become more aware of the benefits and options available, the demand for both 2 Root Type and 6 Root Type Levonorgestrel implants is expected to grow, contributing to the overall expansion of the Global Levonorgestrel Implant Market.
Age 20~24 Years Old, Age 25-34 Years Old, Age 35~44 Years Old, Other in the Global Levonorgestrel Implant Market:
The usage of Levonorgestrel implants varies across different age groups, reflecting the diverse contraceptive needs and preferences of women at different stages of their reproductive lives. For women aged 20-24 years old, Levonorgestrel implants offer a reliable and long-term contraceptive solution that aligns with their active lifestyles and future family planning goals. This age group often seeks contraception that requires minimal daily attention, making implants an attractive option. The implants provide peace of mind and allow young women to focus on their education, careers, and personal development without the constant worry of unintended pregnancies. For women aged 25-34 years old, Levonorgestrel implants continue to be a popular choice as they balance their professional and personal lives. This age group may be considering starting or expanding their families in the future, and the reversible nature of the implants provides flexibility. The long-term protection offered by the implants ensures that they can plan their pregnancies according to their timelines. Women aged 35-44 years old also benefit from the use of Levonorgestrel implants, especially those who have completed their families and are looking for a reliable contraceptive method to avoid further pregnancies. The implants offer a hassle-free solution that fits well with their busy lives, allowing them to focus on their careers, families, and other responsibilities. Additionally, the implants can be a suitable option for women who may have health concerns that make other forms of contraception less ideal. Other age groups, including teenagers and women over 45, may also use Levonorgestrel implants based on individual health needs and personal preferences. The versatility and effectiveness of these implants make them a valuable contraceptive option for women of all ages, contributing to better reproductive health and family planning outcomes.
Global Levonorgestrel Implant Market Outlook:
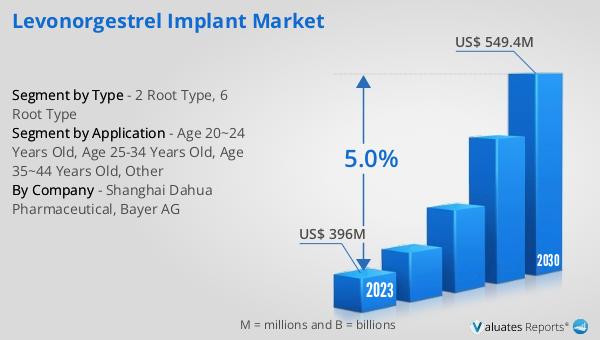
The global Levonorgestrel Implant market was valued at US$ 396 million in 2023 and is anticipated to reach US$ 549.4 million by 2030, witnessing a CAGR of 5.0% during the forecast period from 2024 to 2030. This growth reflects the increasing demand for effective and long-term contraceptive solutions. In the broader context, the global pharmaceutical market was valued at 1475 billion USD in 2022 and is expected to grow at a CAGR of 5% over the next six years. This indicates a robust expansion in the pharmaceutical sector, driven by advancements in medical technology, increasing healthcare expenditure, and rising awareness about health and wellness. In comparison, the chemical drug market is estimated to have grown from 1005 billion USD in 2018 to 1094 billion USD in 2022. This growth trajectory highlights the significant role of pharmaceutical innovations, including contraceptive implants, in addressing global health challenges. The Levonorgestrel Implant market, as a part of this larger pharmaceutical landscape, is poised to contribute to the overall growth by providing reliable and convenient contraceptive options to women worldwide.
| Report Metric | Details |
| Report Name | Levonorgestrel Implant Market |
| Accounted market size in 2023 | US$ 396 million |
| Forecasted market size in 2030 | US$ 549.4 million |
| CAGR | 5.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Shanghai Dahua Pharmaceutical, Bayer AG |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |