What is Global Point-of-care Troponin Testing Instrument Market?
The Global Point-of-care Troponin Testing Instrument Market is a specialized segment within the broader medical diagnostics industry, focusing on devices that enable rapid and accurate testing for cardiac troponin levels at the point of care. Troponin is a protein found in heart muscle, and its presence in the blood is a critical marker for diagnosing heart attacks and other cardiac-related conditions. The market for these instruments is driven by the increasing prevalence of cardiovascular diseases, which are among the leading causes of mortality worldwide. Point-of-care testing (POCT) allows for quicker decision-making in clinical settings, as it provides immediate results compared to traditional laboratory testing, which can take several hours. This immediacy is crucial in emergency situations where time is of the essence. The market encompasses a variety of devices, including desktop and portable instruments, each designed to meet the specific needs of different healthcare settings. As healthcare systems globally strive to improve patient outcomes and reduce hospital stays, the demand for efficient and reliable point-of-care testing solutions continues to grow. This market is characterized by ongoing technological advancements, regulatory considerations, and a competitive landscape with numerous players striving to innovate and capture market share.
Desktop, Portable in the Global Point-of-care Troponin Testing Instrument Market:
In the Global Point-of-care Troponin Testing Instrument Market, devices are broadly categorized into desktop and portable instruments, each serving distinct purposes and settings. Desktop instruments are typically larger and designed for use in settings where space and infrastructure are not constraints, such as hospitals and large diagnostic laboratories. These devices often offer higher throughput, meaning they can process a larger number of tests simultaneously, which is beneficial in high-volume environments. Desktop instruments are generally more robust and may offer a wider range of testing capabilities beyond troponin, making them versatile tools in comprehensive diagnostic settings. On the other hand, portable instruments are compact, lightweight, and designed for use in a variety of settings, including ambulances, remote clinics, and even at the patient's bedside. The portability of these devices allows for immediate testing and results, which is particularly advantageous in emergency situations or in locations where access to full laboratory facilities is limited. Portable instruments are often battery-operated and designed for ease of use, requiring minimal training for healthcare providers. This makes them ideal for use in rural or underserved areas where healthcare resources are scarce. Both desktop and portable instruments are integral to the point-of-care testing market, each offering unique advantages that cater to different healthcare needs. The choice between desktop and portable devices often depends on factors such as the volume of tests required, the setting in which they will be used, and the specific needs of the patient population being served. As technology continues to advance, the line between desktop and portable devices is becoming increasingly blurred, with many manufacturers striving to combine the high throughput of desktop instruments with the convenience and flexibility of portable devices. This convergence is driving innovation in the market, leading to the development of hybrid devices that offer the best of both worlds. These advancements are expected to further enhance the capabilities of point-of-care testing, making it an even more integral part of modern healthcare delivery.
Hospitals, Diagnostic Laboratories, Others in the Global Point-of-care Troponin Testing Instrument Market:
The usage of Global Point-of-care Troponin Testing Instruments spans various healthcare settings, including hospitals, diagnostic laboratories, and other facilities, each with unique requirements and benefits. In hospitals, these instruments are crucial in emergency departments and intensive care units, where rapid diagnosis of cardiac events is essential. The ability to quickly measure troponin levels at the point of care allows healthcare providers to make timely decisions regarding patient management, potentially improving outcomes and reducing the length of hospital stays. In emergency situations, where every minute counts, point-of-care testing can significantly impact the speed and accuracy of diagnosis, enabling prompt treatment and intervention. Diagnostic laboratories, while traditionally associated with centralized testing, are increasingly incorporating point-of-care instruments to complement their existing capabilities. These instruments allow laboratories to offer rapid testing services, which can be particularly beneficial for outpatient clinics and smaller healthcare facilities that may not have the resources for full-scale laboratory operations. By providing quick and reliable results, point-of-care testing can enhance the overall efficiency of diagnostic services, leading to faster patient turnaround and improved satisfaction. Beyond hospitals and laboratories, point-of-care troponin testing instruments are also used in a variety of other settings, including ambulatory care centers, urgent care clinics, and even in-home healthcare scenarios. In these environments, the portability and ease of use of these devices are significant advantages, allowing healthcare providers to deliver high-quality care in diverse and often challenging conditions. For instance, in rural or remote areas where access to healthcare facilities is limited, portable point-of-care testing instruments can provide critical diagnostic capabilities, bridging the gap between patients and necessary medical services. Additionally, in-home testing can empower patients to take a more active role in managing their health, particularly for those with chronic conditions that require regular monitoring. Overall, the versatility and adaptability of point-of-care troponin testing instruments make them invaluable tools across a wide range of healthcare settings, contributing to improved patient care and outcomes.
Global Point-of-care Troponin Testing Instrument Market Outlook:
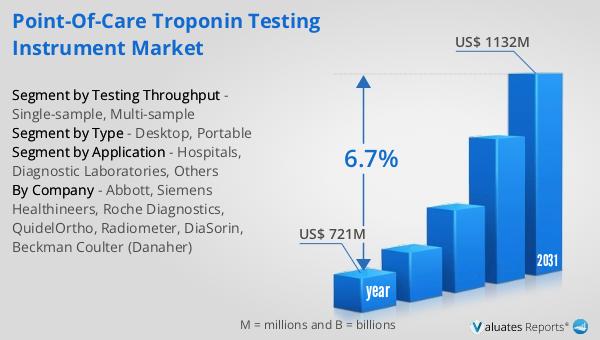
The outlook for the Global Point-of-care Troponin Testing Instrument Market is promising, with significant growth anticipated over the coming years. In 2024, the market was valued at approximately US$ 721 million, reflecting the increasing demand for rapid and accurate cardiac diagnostics. By 2031, the market is projected to expand to a revised size of US$ 1132 million, driven by a compound annual growth rate (CAGR) of 6.7% during the forecast period. This growth is indicative of the rising prevalence of cardiovascular diseases and the corresponding need for efficient diagnostic solutions. The ability to quickly and accurately measure troponin levels at the point of care is becoming increasingly important as healthcare systems worldwide strive to improve patient outcomes and reduce the burden on hospital resources. The market's expansion is also fueled by ongoing technological advancements, which are enhancing the capabilities and accessibility of point-of-care testing instruments. As these devices become more sophisticated and user-friendly, their adoption is expected to increase across various healthcare settings, from large hospitals to remote clinics. The competitive landscape of the market is characterized by numerous players, each striving to innovate and capture market share through the development of new and improved testing solutions. This dynamic environment is likely to drive further advancements in the field, ultimately benefiting patients and healthcare providers alike. Overall, the Global Point-of-care Troponin Testing Instrument Market is poised for significant growth, reflecting the critical role these devices play in modern healthcare delivery.
| Report Metric | Details |
| Report Name | Point-of-care Troponin Testing Instrument Market |
| Accounted market size in year | US$ 721 million |
| Forecasted market size in 2031 | US$ 1132 million |
| CAGR | 6.7% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Testing Throughput |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Abbott, Siemens Healthineers, Roche Diagnostics, QuidelOrtho, Radiometer, DiaSorin, Beckman Coulter (Danaher) |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
