What is Global Chlorphenamine Maleate API Market?
The Global Chlorphenamine Maleate API Market is a significant segment within the pharmaceutical industry, focusing on the production and distribution of the active pharmaceutical ingredient (API) used in various antihistamine medications. Chlorphenamine Maleate is primarily used to treat allergic reactions, hay fever, and the common cold by alleviating symptoms such as runny nose, sneezing, and itching. The market for this API is driven by the increasing prevalence of allergies and the growing demand for effective over-the-counter and prescription medications. The market encompasses a wide range of activities, including the synthesis of the API, quality control, regulatory compliance, and distribution to pharmaceutical companies worldwide. As a critical component in the formulation of antihistamine drugs, the Chlorphenamine Maleate API market plays a vital role in ensuring the availability of effective treatments for allergy sufferers globally. The market is characterized by a competitive landscape with several key players involved in the production and supply of this API, each striving to maintain high standards of purity and efficacy in their products. The ongoing research and development efforts in this field aim to enhance the therapeutic benefits of Chlorphenamine Maleate, ensuring its continued relevance in the pharmaceutical industry.
Purity :No Higher Than 98%, Purity :Higher Than 98% in the Global Chlorphenamine Maleate API Market:
In the Global Chlorphenamine Maleate API Market, the purity of the product is a crucial factor that significantly influences its application and effectiveness. The market is broadly categorized into two segments based on purity levels: those with purity no higher than 98% and those with purity higher than 98%. Products with purity no higher than 98% are typically used in applications where ultra-high purity is not a critical requirement. These applications may include certain over-the-counter medications where the cost-effectiveness of the product is a priority. The slightly lower purity level does not significantly impact the therapeutic efficacy for general use, making it a viable option for mass-market products. On the other hand, Chlorphenamine Maleate API with purity higher than 98% is preferred in applications that demand stringent quality standards, such as prescription medications and formulations requiring precise dosing and minimal impurities. The higher purity level ensures that the API meets the rigorous standards set by regulatory bodies, providing assurance of safety and efficacy for end-users. This segment is particularly important in the production of medications for sensitive populations, such as children and the elderly, where the margin for error is minimal. The distinction between these two purity levels also reflects the varying manufacturing processes and quality control measures employed by producers. Manufacturers of higher purity Chlorphenamine Maleate API often invest in advanced technologies and stringent quality assurance protocols to achieve the desired purity levels. This investment is reflected in the pricing of the product, with higher purity APIs typically commanding a premium in the market. The choice between these two purity levels is influenced by several factors, including the intended application, regulatory requirements, and cost considerations. Pharmaceutical companies must carefully evaluate these factors when selecting the appropriate Chlorphenamine Maleate API for their products. The global market for Chlorphenamine Maleate API is thus characterized by a dynamic interplay between purity levels, application requirements, and regulatory standards, each contributing to the overall landscape of the industry. As the demand for effective antihistamine treatments continues to grow, the importance of maintaining high purity standards in the production of Chlorphenamine Maleate API cannot be overstated. This focus on purity ensures that the API remains a reliable and effective component in the formulation of medications that improve the quality of life for allergy sufferers worldwide.
Tablets, Injection in the Global Chlorphenamine Maleate API Market:
The usage of Global Chlorphenamine Maleate API in the formulation of tablets and injections highlights its versatility and importance in the pharmaceutical industry. Tablets are one of the most common forms of medication delivery, offering convenience and ease of use for patients. Chlorphenamine Maleate API is used in the production of antihistamine tablets that are widely available both over-the-counter and by prescription. These tablets are designed to provide relief from allergy symptoms such as sneezing, runny nose, and itching. The formulation of tablets involves precise dosing of the API to ensure consistent therapeutic effects, making the purity and quality of the API critical factors in the manufacturing process. The production of tablets also involves considerations of stability, shelf life, and patient compliance, all of which are influenced by the characteristics of the Chlorphenamine Maleate API used. Injections, on the other hand, represent a more specialized application of Chlorphenamine Maleate API, typically used in clinical settings where rapid onset of action is required. Injectable formulations are often used in cases of severe allergic reactions or when oral administration is not feasible. The use of Chlorphenamine Maleate API in injections requires adherence to stringent quality standards to ensure safety and efficacy. The API must be of high purity to minimize the risk of adverse reactions and to ensure that the medication delivers the desired therapeutic effects promptly. The formulation of injectable medications also involves considerations of sterility, stability, and compatibility with other components, all of which are critical to the success of the treatment. The choice between tablets and injections as a delivery method for Chlorphenamine Maleate API is influenced by several factors, including the severity of the condition being treated, patient preferences, and the clinical setting. Tablets offer a convenient and non-invasive option for managing mild to moderate allergy symptoms, while injections provide a rapid and effective solution for more severe cases. The versatility of Chlorphenamine Maleate API in these different formulations underscores its importance as a key component in the treatment of allergic conditions. As the demand for effective allergy treatments continues to grow, the role of Chlorphenamine Maleate API in the production of tablets and injections remains critical to meeting the needs of patients worldwide. The ongoing development and refinement of these formulations ensure that patients have access to safe, effective, and convenient treatment options for managing their allergy symptoms.
Global Chlorphenamine Maleate API Market Outlook:
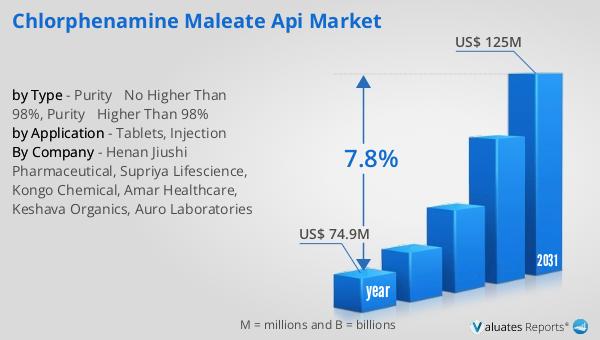
The global market for Chlorphenamine Maleate API was valued at $74.9 million in 2024 and is anticipated to expand to a revised size of $125 million by 2031, reflecting a compound annual growth rate (CAGR) of 7.8% during the forecast period. The market is dominated by the top five manufacturers, which include Henan Jiushi Pharmaceutical, Supriya Lifescience, Kongo Chemical, Amar Healthcare, and Auro Laboratories, collectively holding about 60% of the market share. Among these, Henan Jiushi Pharmaceutical stands out as the largest manufacturer, commanding over 30% of the market share. India emerges as the most significant production hub for Chlorphenamine Maleate API globally, contributing more than 45% to the market share. In terms of application, tablets account for approximately 60% of the market share, highlighting their prominence as a preferred delivery method for this API. This market outlook underscores the competitive landscape and the strategic importance of key players and regions in shaping the future of the Chlorphenamine Maleate API market. The growth trajectory of this market is driven by the increasing demand for effective antihistamine treatments and the ongoing efforts of manufacturers to enhance the quality and availability of Chlorphenamine Maleate API.
| Report Metric | Details |
| Report Name | Chlorphenamine Maleate API Market |
| Accounted market size in year | US$ 74.9 million |
| Forecasted market size in 2031 | US$ 125 million |
| CAGR | 7.8% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Henan Jiushi Pharmaceutical, Supriya Lifescience, Kongo Chemical, Amar Healthcare, Keshava Organics, Auro Laboratories |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |