What is Global Pharmaceutical Stability Testing Service Market?
The Global Pharmaceutical Stability Testing Service Market is a crucial segment within the pharmaceutical industry, focusing on ensuring the safety, efficacy, and quality of pharmaceutical products over time. Stability testing is a scientific process that evaluates how environmental factors such as temperature, humidity, and light affect a drug's quality. This market encompasses various services that help pharmaceutical companies comply with regulatory requirements and ensure their products remain effective throughout their shelf life. The demand for stability testing services is driven by the need for new drug development, stringent regulatory guidelines, and the increasing complexity of pharmaceutical formulations. Companies in this market offer a range of services, including long-term, accelerated, and stress testing, to assess the stability of active pharmaceutical ingredients (APIs) and finished products. As the pharmaceutical industry continues to grow and innovate, the stability testing service market plays a vital role in supporting the development of safe and effective medications for global consumption.
Long-term Stability Testing Services, Accelerated Stability Testing Services in the Global Pharmaceutical Stability Testing Service Market:
Long-term Stability Testing Services and Accelerated Stability Testing Services are two critical components of the Global Pharmaceutical Stability Testing Service Market. Long-term stability testing involves evaluating a drug's stability under recommended storage conditions over an extended period, typically ranging from one to five years. This type of testing is essential for determining the shelf life of pharmaceutical products and ensuring they remain safe and effective for consumers. It involves storing the drug at specific conditions and periodically analyzing its physical, chemical, and microbiological properties. The data obtained from long-term stability testing is crucial for regulatory submissions and helps pharmaceutical companies establish expiration dates and storage guidelines for their products. On the other hand, Accelerated Stability Testing Services are designed to speed up the stability evaluation process by subjecting the drug to elevated stress conditions, such as higher temperatures and humidity levels. This approach helps predict the drug's shelf life in a shorter time frame, typically six months to a year. Accelerated testing is particularly useful during the early stages of drug development when quick decisions are needed to move forward with clinical trials or product launches. By simulating the effects of time under harsher conditions, accelerated stability testing provides valuable insights into the potential degradation pathways of a drug and helps identify any formulation issues that may arise. Both long-term and accelerated stability testing services are integral to the pharmaceutical industry's efforts to ensure product quality and compliance with regulatory standards. They provide essential data that supports the development, approval, and commercialization of new drugs, ultimately contributing to the availability of safe and effective medications for patients worldwide.
Pharmaceutical, Laboratory, Other in the Global Pharmaceutical Stability Testing Service Market:
The Global Pharmaceutical Stability Testing Service Market finds its application in various areas, including pharmaceuticals, laboratories, and other sectors. In the pharmaceutical industry, stability testing is a fundamental part of the drug development process. It ensures that pharmaceutical products maintain their intended quality, safety, and efficacy throughout their shelf life. Stability testing data is required for regulatory submissions and helps pharmaceutical companies establish expiration dates, storage conditions, and packaging requirements for their products. This information is crucial for ensuring that patients receive safe and effective medications. In laboratories, stability testing services are used to support research and development activities. Laboratories conduct stability studies to evaluate the effects of environmental factors on the stability of active pharmaceutical ingredients (APIs) and finished products. This data is essential for optimizing formulations, selecting appropriate packaging materials, and ensuring the overall quality of pharmaceutical products. Additionally, stability testing services are used in other sectors, such as cosmetics and food industries, where product stability is equally important. In these industries, stability testing helps ensure that products remain safe and effective for consumers throughout their shelf life. Overall, the Global Pharmaceutical Stability Testing Service Market plays a vital role in supporting the development and commercialization of safe and effective products across various industries.
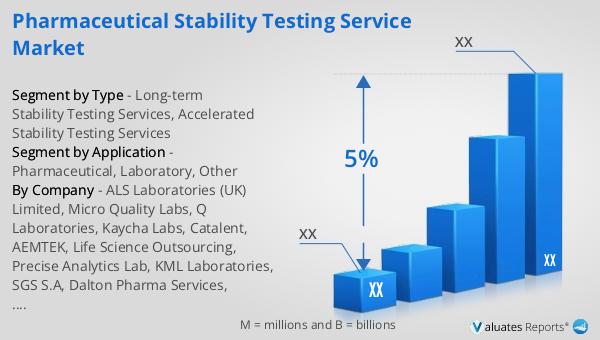
Global Pharmaceutical Stability Testing Service Market Outlook:
The outlook for the Global Pharmaceutical Stability Testing Service Market is closely tied to the broader pharmaceutical market trends. In 2022, the global pharmaceutical market was valued at approximately 1,475 billion USD, with an expected compound annual growth rate (CAGR) of 5% over the next six years. This growth is driven by factors such as increasing demand for innovative drugs, advancements in biotechnology, and the rising prevalence of chronic diseases. In comparison, the chemical drug market, a subset of the pharmaceutical industry, was estimated to grow from 1,005 billion USD in 2018 to 1,094 billion USD in 2022. This growth highlights the ongoing demand for chemical-based pharmaceuticals and the need for stability testing services to ensure their quality and efficacy. As the pharmaceutical industry continues to expand, the demand for stability testing services is expected to increase, supporting the development and commercialization of safe and effective medications. The Global Pharmaceutical Stability Testing Service Market is poised to play a crucial role in ensuring the quality and safety of pharmaceutical products, ultimately benefiting patients and consumers worldwide.
| Report Metric | Details |
| Report Name | Pharmaceutical Stability Testing Service Market |
| CAGR | 5% |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | ALS Laboratories (UK) Limited, Micro Quality Labs, Q Laboratories, Kaycha Labs, Catalent, AEMTEK, Life Science Outsourcing, Precise Analytics Lab, KML Laboratories, SGS S.A, Dalton Pharma Services, Rockland Immunochemicals, Charles River Laboratories, Weiss Technik North America, Inc, Impact Analytical, STILLMEADOW, Eurofins BioPharma, Microchem Laboratory, Pace Analytical Life Sciences, Tepnel Pharma Services, Kappa Laboratories, FreeThink Technologies, Nelson Labs Europe, NANOLAB |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |