What is Global Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market?
The Global Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market is a specialized segment within the broader chemical industry, focusing on the production and distribution of high-purity CMC for pharmaceutical applications. Sodium Carboxymethyl Cellulose is a cellulose derivative that is widely used in the pharmaceutical industry due to its excellent properties as a thickener, stabilizer, and binder. This market is driven by the increasing demand for pharmaceutical products that require high-quality excipients to ensure the efficacy and safety of medications. Pharmaceutical grade CMC is used in various formulations, including tablets, capsules, and liquid suspensions, where it acts as a binder, disintegrant, or viscosity enhancer. The market is characterized by stringent regulatory standards, as the product must meet specific purity and quality criteria to be suitable for pharmaceutical use. Key players in this market are continuously investing in research and development to improve the quality and functionality of CMC, as well as to expand their product offerings to meet the evolving needs of the pharmaceutical industry. The market's growth is also supported by the increasing prevalence of chronic diseases, which drives the demand for effective and reliable pharmaceutical formulations.
USP, BP, Other in the Global Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market:
In the Global Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market, the terms USP, BP, and Other refer to different standards and specifications that the product must meet to be considered suitable for pharmaceutical use. USP stands for the United States Pharmacopeia, which is a compendium of drug standards used in the United States. It sets the quality, purity, strength, and consistency standards for medicines, food ingredients, and dietary supplements. Pharmaceutical grade CMC that meets USP standards is recognized for its high quality and is widely used in the U.S. pharmaceutical industry. BP, on the other hand, stands for the British Pharmacopoeia, which is the official collection of standards for UK medicinal products and pharmaceutical substances. BP standards are used to ensure the quality and safety of medicines in the UK and other countries that recognize BP standards. CMC that complies with BP standards is considered to be of high quality and is used in pharmaceutical formulations in these regions. The "Other" category includes CMC products that meet different regional or international standards, such as the European Pharmacopoeia (EP) or the Japanese Pharmacopoeia (JP). These standards are essential for ensuring that pharmaceutical grade CMC is safe and effective for use in various drug formulations. The adherence to these standards is crucial for manufacturers, as it ensures that their products can be used in different markets around the world. Each of these standards has specific requirements for the physical and chemical properties of CMC, such as viscosity, degree of substitution, and purity levels. Manufacturers must conduct rigorous testing and quality control processes to ensure that their products meet these standards. This often involves sophisticated analytical techniques and equipment to verify the compliance of CMC with the required specifications. The choice of standard depends on the target market and the regulatory requirements of the region where the pharmaceutical product will be sold. For instance, a pharmaceutical company targeting the U.S. market would prioritize CMC that meets USP standards, while a company focusing on the European market might prefer CMC that complies with EP standards. The ability to meet multiple standards can be a competitive advantage for manufacturers, as it allows them to cater to a broader range of customers and markets. In addition to meeting these standards, manufacturers must also consider other factors such as cost, availability, and supply chain logistics when selecting CMC for their pharmaceutical formulations. The global nature of the pharmaceutical industry means that manufacturers often need to source CMC from multiple suppliers to ensure a consistent and reliable supply. This requires careful coordination and management to ensure that all suppliers meet the necessary quality and regulatory standards. Overall, the adherence to USP, BP, and other standards is a critical aspect of the Global Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market, as it ensures the safety, efficacy, and quality of pharmaceutical products that contain CMC as an excipient.
Coating Agent, Tablet Binder, Suspending Agent, Tackifier, Others in the Global Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market:
The Global Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market finds its application in various areas within the pharmaceutical industry, each serving a specific purpose to enhance the quality and effectiveness of pharmaceutical products. As a coating agent, CMC is used to provide a protective layer on tablets and capsules, which helps in masking the taste of the active ingredients, improving the appearance of the product, and controlling the release of the drug. The coating also protects the drug from environmental factors such as moisture and light, which can degrade the active ingredients. In this role, CMC's film-forming properties are highly valued, as they contribute to the stability and shelf-life of the pharmaceutical product. As a tablet binder, CMC plays a crucial role in ensuring that the ingredients in a tablet stick together, providing the necessary mechanical strength to withstand handling and transportation. The binding properties of CMC help in maintaining the integrity of the tablet, preventing it from breaking apart during manufacturing, packaging, and consumption. This is particularly important for tablets that contain multiple active ingredients, as it ensures that each dose contains the correct amount of each component. CMC's ability to absorb water and swell makes it an effective binder, as it helps in the compaction and cohesion of the tablet ingredients. As a suspending agent, CMC is used in liquid pharmaceutical formulations to keep the active ingredients evenly distributed throughout the solution. This is essential for ensuring that each dose contains the correct amount of active ingredient, as settling or separation of the ingredients can lead to inconsistent dosing. CMC's ability to increase the viscosity of a solution helps in maintaining the uniform distribution of the particles, preventing them from settling at the bottom of the container. This property is particularly important for suspensions that are intended for oral or topical administration, as it ensures the efficacy and safety of the product. As a tackifier, CMC is used in transdermal drug delivery systems, where it helps in adhering the drug-containing patch to the skin. The tackifying properties of CMC ensure that the patch remains in place for the required duration, allowing for the controlled release of the drug through the skin. This is important for maintaining the therapeutic effect of the drug, as any displacement or removal of the patch can lead to a reduction in the drug's efficacy. CMC's biocompatibility and non-irritating nature make it an ideal choice for use in transdermal systems, as it minimizes the risk of skin irritation or allergic reactions. In addition to these specific applications, CMC is also used in other areas of the pharmaceutical industry, such as in the formulation of gels, creams, and ointments. Its thickening and stabilizing properties make it a valuable ingredient in these products, as it helps in achieving the desired consistency and texture. CMC's ability to form a gel-like structure when mixed with water is particularly useful in topical formulations, as it allows for easy application and absorption of the active ingredients. Overall, the versatility and functionality of CMC make it an indispensable excipient in the pharmaceutical industry, contributing to the development of safe, effective, and high-quality pharmaceutical products.
Global Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market Outlook:
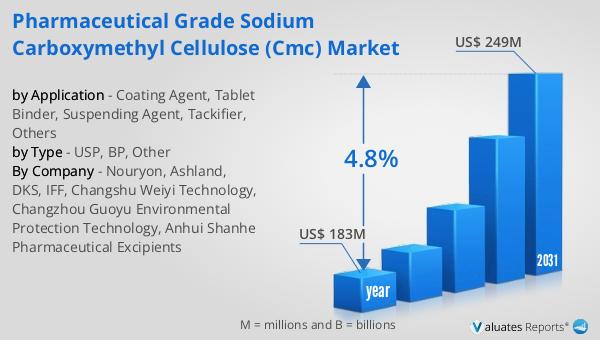
In 2024, the global market for Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) was valued at approximately $183 million. This market is anticipated to experience growth over the coming years, with projections indicating that it will reach an estimated size of $249 million by 2031. This growth trajectory represents a compound annual growth rate (CAGR) of 4.8% during the forecast period. The increasing demand for high-quality pharmaceutical excipients, driven by the rising prevalence of chronic diseases and the need for effective drug formulations, is a key factor contributing to this market expansion. Pharmaceutical Grade CMC is widely used in various drug formulations due to its excellent properties as a binder, thickener, and stabilizer. The market's growth is also supported by advancements in pharmaceutical manufacturing processes and the development of new drug delivery systems that require high-purity excipients. As pharmaceutical companies continue to innovate and develop new products, the demand for reliable and effective excipients like CMC is expected to rise. Additionally, the stringent regulatory standards governing the quality and safety of pharmaceutical products further emphasize the importance of using high-grade excipients, which in turn drives the demand for Pharmaceutical Grade CMC. The market's growth is also influenced by the increasing focus on personalized medicine and the development of targeted drug delivery systems, which require specialized excipients to achieve the desired therapeutic outcomes. Overall, the Global Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market is poised for steady growth, driven by the ongoing advancements in the pharmaceutical industry and the increasing demand for high-quality excipients.
| Report Metric | Details |
| Report Name | Pharmaceutical Grade Sodium Carboxymethyl Cellulose (CMC) Market |
| Accounted market size in year | US$ 183 million |
| Forecasted market size in 2031 | US$ 249 million |
| CAGR | 4.8% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Nouryon, Ashland, DKS, IFF, Changshu Weiyi Technology, Changzhou Guoyu Environmental Protection Technology, Anhui Shanhe Pharmaceutical Excipients |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |