What is Global Cloperastine Hydrochloride API Market?
The Global Cloperastine Hydrochloride API Market is a specialized segment within the pharmaceutical industry, focusing on the production and distribution of Cloperastine Hydrochloride, an active pharmaceutical ingredient (API) primarily used in cough suppressants. This market is driven by the demand for effective treatments for respiratory conditions, particularly those involving persistent coughs. Cloperastine Hydrochloride works by acting on the central nervous system to suppress the cough reflex, providing relief to patients. The market encompasses various stages of the supply chain, including raw material procurement, API manufacturing, and distribution to pharmaceutical companies that formulate the final medicinal products. The growth of this market is influenced by factors such as increasing incidences of respiratory diseases, advancements in pharmaceutical manufacturing technologies, and the expansion of healthcare infrastructure in emerging economies. Additionally, regulatory approvals and quality standards play a crucial role in shaping the market dynamics, as compliance with international guidelines ensures the safety and efficacy of the API. Overall, the Global Cloperastine Hydrochloride API Market is an essential component of the broader pharmaceutical landscape, contributing to the development of effective therapeutic solutions for respiratory health.
≥98.0%, ≥95.0% in the Global Cloperastine Hydrochloride API Market:
In the Global Cloperastine Hydrochloride API Market, the purity levels of the API are critical factors that determine its application and efficacy. Two common purity specifications are ≥98.0% and ≥95.0%, which indicate the percentage of Cloperastine Hydrochloride present in the API compared to other substances. A purity level of ≥98.0% is often preferred for pharmaceutical applications where high efficacy and safety are paramount. This level of purity ensures that the API is free from significant impurities that could affect the drug's performance or cause adverse effects in patients. Pharmaceutical companies that prioritize high-quality standards typically opt for APIs with this level of purity to maintain the integrity of their products and comply with stringent regulatory requirements. On the other hand, a purity level of ≥95.0% may be suitable for certain research applications where the focus is on understanding the API's properties or developing new formulations. In research settings, slight variations in purity may be acceptable, provided they do not compromise the study's objectives or outcomes. The choice between these purity levels depends on the intended use of the API, the regulatory landscape, and the specific requirements of the end product. Manufacturers in the Global Cloperastine Hydrochloride API Market must carefully consider these factors when producing and supplying the API to ensure it meets the needs of their clients and adheres to industry standards. The production process for achieving these purity levels involves advanced techniques such as crystallization, filtration, and chromatography, which help isolate and purify the desired compound. Quality control measures, including rigorous testing and validation, are essential to confirm that the API meets the specified purity criteria. This involves analytical methods like high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS), which provide precise measurements of the API's composition. The demand for high-purity Cloperastine Hydrochloride APIs is driven by the need for effective and safe medications, particularly in the treatment of respiratory conditions. As healthcare systems worldwide continue to evolve, the emphasis on quality and efficacy in pharmaceutical products remains a top priority. This underscores the importance of maintaining high purity standards in the production of APIs, as they form the foundation of effective therapeutic solutions. Furthermore, the competitive landscape of the Global Cloperastine Hydrochloride API Market is shaped by the ability of manufacturers to consistently deliver high-quality products that meet the diverse needs of their clients. Companies that invest in state-of-the-art manufacturing facilities and adopt best practices in quality assurance are better positioned to succeed in this market. Additionally, collaborations and partnerships with research institutions and pharmaceutical companies can enhance the development and application of Cloperastine Hydrochloride APIs, fostering innovation and expanding the market's potential. In conclusion, the purity levels of Cloperastine Hydrochloride APIs play a crucial role in determining their suitability for various applications within the pharmaceutical and research sectors. By adhering to high purity standards and implementing robust quality control measures, manufacturers can ensure the safety and efficacy of their products, ultimately contributing to the advancement of healthcare solutions for respiratory health.
Pharmaceutical, Research in the Global Cloperastine Hydrochloride API Market:
The Global Cloperastine Hydrochloride API Market finds significant usage in the pharmaceutical and research sectors, each with distinct applications and requirements. In the pharmaceutical industry, Cloperastine Hydrochloride is primarily used as an active ingredient in cough suppressants. Its ability to effectively reduce the cough reflex makes it a valuable component in medications designed to alleviate symptoms associated with respiratory conditions such as chronic bronchitis, asthma, and the common cold. Pharmaceutical companies utilize Cloperastine Hydrochloride APIs to formulate over-the-counter and prescription medications that provide relief to patients experiencing persistent coughs. The API's efficacy and safety profile are critical considerations for pharmaceutical manufacturers, who must ensure that their products meet regulatory standards and deliver the intended therapeutic benefits. This involves rigorous testing and validation processes to confirm the API's purity, potency, and stability, as well as compliance with international quality guidelines. In the research sector, Cloperastine Hydrochloride APIs are used to explore new therapeutic applications and improve existing formulations. Researchers may investigate the API's pharmacological properties, mechanisms of action, and potential interactions with other compounds to gain a deeper understanding of its therapeutic potential. This research can lead to the development of novel drug formulations or the identification of new indications for Cloperastine Hydrochloride, expanding its application beyond traditional cough suppression. Additionally, research involving Cloperastine Hydrochloride APIs may focus on optimizing manufacturing processes to enhance the API's purity, yield, and cost-effectiveness. This can involve exploring alternative synthesis routes, refining purification techniques, or implementing advanced analytical methods to improve quality control. Collaborative efforts between research institutions and pharmaceutical companies can drive innovation in the Global Cloperastine Hydrochloride API Market, leading to the development of more effective and accessible treatments for respiratory conditions. The integration of cutting-edge technologies, such as computational modeling and high-throughput screening, can further accelerate the discovery and development of new applications for Cloperastine Hydrochloride APIs. By leveraging these tools, researchers can identify promising compounds and optimize their properties for specific therapeutic targets, ultimately contributing to the advancement of respiratory healthcare. In summary, the Global Cloperastine Hydrochloride API Market plays a vital role in both the pharmaceutical and research sectors, providing essential components for the development of effective cough suppressants and facilitating the exploration of new therapeutic applications. The continued focus on quality, innovation, and collaboration within this market is essential for addressing the evolving needs of patients and advancing the field of respiratory medicine.
Global Cloperastine Hydrochloride API Market Outlook:
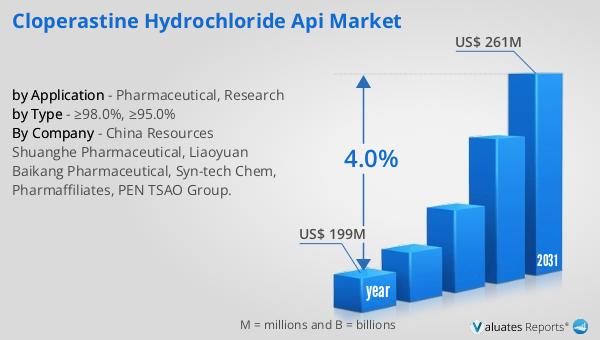
In 2024, the global market for Cloperastine Hydrochloride API was valued at approximately $199 million. This figure represents the total worth of the market, encompassing all aspects of production, distribution, and sales of this specific active pharmaceutical ingredient. Looking ahead, projections indicate that by 2031, the market is expected to grow to a revised size of around $261 million. This anticipated growth reflects a compound annual growth rate (CAGR) of 4.0% over the forecast period. The steady increase in market size can be attributed to several factors, including rising demand for effective cough suppressants, advancements in pharmaceutical manufacturing technologies, and the expansion of healthcare infrastructure in emerging economies. As respiratory conditions continue to affect a significant portion of the global population, the need for reliable and effective treatments remains a priority. The Cloperastine Hydrochloride API Market is poised to meet this demand by providing high-quality APIs that form the basis of therapeutic solutions for cough suppression. Additionally, the market's growth is supported by ongoing research and development efforts aimed at enhancing the efficacy and safety of Cloperastine Hydrochloride-based medications. By investing in innovation and maintaining high standards of quality, manufacturers in this market can capitalize on the growing demand for respiratory health solutions and contribute to the overall advancement of the pharmaceutical industry.
| Report Metric | Details |
| Report Name | Cloperastine Hydrochloride API Market |
| Accounted market size in year | US$ 199 million |
| Forecasted market size in 2031 | US$ 261 million |
| CAGR | 4.0% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| by Type |
|
| by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | China Resources Shuanghe Pharmaceutical, Liaoyuan Baikang Pharmaceutical, Syn-tech Chem, Pharmaffiliates, PEN TSAO Group. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |