What is Global Portable Laboratory Market?
The Global Portable Laboratory Market refers to the industry focused on the development, production, and distribution of portable laboratory equipment. These laboratories are compact, mobile units that can be easily transported and set up in various locations, making them ideal for fieldwork and on-site testing. They are equipped with essential laboratory instruments and tools, allowing scientists, researchers, and technicians to conduct experiments and analyses outside of traditional laboratory settings. The demand for portable laboratories has been growing due to their versatility and convenience, especially in remote areas where access to full-scale laboratory facilities is limited. They are used in a wide range of applications, including environmental monitoring, medical diagnostics, forensic investigations, and educational purposes. The market is driven by advancements in technology, increasing research activities, and the need for rapid and accurate testing solutions in various industries. As a result, the Global Portable Laboratory Market is expected to continue expanding, offering innovative solutions to meet the evolving needs of different sectors.
General, Specialty in the Global Portable Laboratory Market:
The Global Portable Laboratory Market can be broadly categorized into general and specialty segments. General portable laboratories are designed to perform a wide range of basic laboratory functions and are typically used in educational settings, field research, and preliminary testing. These laboratories are equipped with standard instruments such as microscopes, centrifuges, spectrophotometers, and basic chemical analysis tools. They provide a versatile platform for conducting various scientific experiments and analyses, making them suitable for schools, universities, and field researchers who require a flexible and mobile laboratory setup. On the other hand, specialty portable laboratories are tailored for specific applications and industries. These laboratories are equipped with specialized instruments and tools designed to meet the unique requirements of particular fields. For example, portable medical laboratories are equipped with diagnostic equipment for conducting on-site medical tests and screenings, making them invaluable in remote or underserved areas where access to healthcare facilities is limited. Similarly, portable forensic laboratories are designed for crime scene investigations, equipped with tools for collecting and analyzing evidence on-site. Environmental portable laboratories are used for monitoring and analyzing air, water, and soil samples, providing critical data for environmental assessments and regulatory compliance. The specialty segment also includes portable biosafety laboratories, which are designed to handle hazardous biological materials safely. These laboratories are equipped with advanced containment systems and safety protocols to prevent the spread of infectious agents, making them essential for research and diagnostic work involving dangerous pathogens. The growing demand for portable laboratories in various industries is driven by the need for rapid, accurate, and on-site testing solutions. Advancements in technology have enabled the development of more sophisticated and compact laboratory equipment, enhancing the capabilities and efficiency of portable laboratories. Additionally, the increasing focus on field-based research and the need for real-time data collection and analysis have further fueled the demand for portable laboratories. As a result, both general and specialty portable laboratories are expected to play a crucial role in addressing the evolving needs of different sectors, providing innovative solutions for on-site testing and analysis.
Science Education, Science Research, Air, Water, and Soil Analysis and Monitoring, Biosafety in the Global Portable Laboratory Market:
The Global Portable Laboratory Market finds extensive usage in various areas, including science education, science research, air, water, and soil analysis and monitoring, and biosafety. In science education, portable laboratories provide students with hands-on learning experiences, allowing them to conduct experiments and gain practical knowledge outside of traditional classroom settings. These laboratories are particularly useful in remote or underfunded schools where access to full-scale laboratory facilities is limited. By bringing the laboratory to the students, portable laboratories enhance the quality of science education and inspire the next generation of scientists. In science research, portable laboratories enable researchers to conduct fieldwork and on-site experiments, providing them with the flexibility to collect and analyze data in real-time. This is especially important in environmental research, where scientists need to monitor and analyze air, water, and soil samples in their natural settings. Portable laboratories equipped with advanced analytical instruments allow researchers to obtain accurate and timely data, facilitating better understanding and management of environmental issues. In the field of air, water, and soil analysis and monitoring, portable laboratories play a critical role in assessing environmental quality and ensuring regulatory compliance. These laboratories are used by environmental agencies, industries, and researchers to conduct on-site testing and analysis of pollutants and contaminants. By providing rapid and accurate results, portable laboratories help in identifying and addressing environmental hazards, protecting public health, and preserving natural resources. In the area of biosafety, portable laboratories are essential for handling and analyzing hazardous biological materials safely. These laboratories are equipped with advanced containment systems and safety protocols to prevent the spread of infectious agents, making them crucial for research and diagnostic work involving dangerous pathogens. Portable biosafety laboratories are used in various settings, including hospitals, research institutions, and field operations, to conduct on-site testing and analysis of infectious diseases. They provide a safe and controlled environment for handling and studying pathogens, contributing to the prevention and control of infectious disease outbreaks. Overall, the Global Portable Laboratory Market offers versatile and innovative solutions for various applications, enhancing the capabilities of scientists, researchers, and educators in conducting on-site testing and analysis.
Global Portable Laboratory Market Outlook:
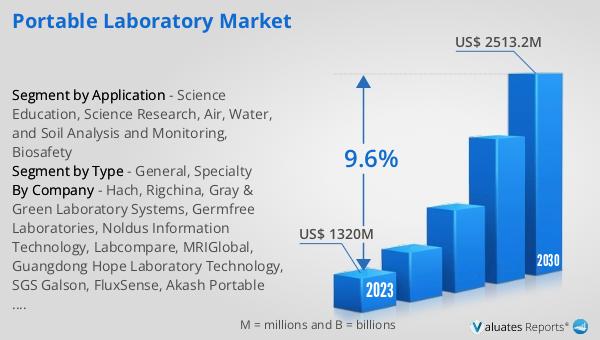
The global Portable Laboratory market was valued at US$ 1320 million in 2023 and is anticipated to reach US$ 2513.2 million by 2030, witnessing a CAGR of 9.6% during the forecast period from 2024 to 2030. This significant growth reflects the increasing demand for portable laboratories across various sectors, driven by the need for rapid, accurate, and on-site testing solutions. The market's expansion is supported by advancements in technology, which have enabled the development of more sophisticated and compact laboratory equipment. These innovations have enhanced the capabilities and efficiency of portable laboratories, making them indispensable tools for fieldwork, research, and diagnostics. The growing focus on field-based research, environmental monitoring, and real-time data collection has further fueled the demand for portable laboratories. As industries and research institutions continue to seek flexible and mobile laboratory solutions, the Global Portable Laboratory Market is expected to witness sustained growth, providing innovative and practical solutions to meet the evolving needs of various sectors.
| Report Metric | Details |
| Report Name | Portable Laboratory Market |
| Accounted market size in 2023 | US$ 1320 million |
| Forecasted market size in 2030 | US$ 2513.2 million |
| CAGR | 9.6% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Hach, Rigchina, Gray & Green Laboratory Systems, Germfree Laboratories, Noldus Information Technology, Labcompare, MRIGlobal, Guangdong Hope Laboratory Technology, SGS Galson, FluxSense, Akash Portable Cabins, Testmak, Palintest, Biomedhelix, Starrco, DENIOS, Hemco, Sepor, Odulair, Symbiote, Biologics Modular, A-1 Rentals, Century Industries, Apex Instruments, Clegg Industries, Technical Furniture Group |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |