What is Influenza A Self-test Kits - Global Market?
Influenza A self-test kits are innovative medical devices designed to allow individuals to test themselves for the presence of the Influenza A virus in the comfort of their own homes. These kits have gained significant attention in the global market due to their convenience, speed, and ease of use. Typically, these kits include a swab for collecting a sample from the nasal passage, a test strip or cassette, and a reagent solution. The user follows simple instructions to collect the sample, apply it to the test strip, and wait for the results, which are usually available within minutes. The growing demand for these kits is driven by the increasing awareness of influenza's impact on public health, the need for rapid diagnosis, and the desire to reduce the burden on healthcare facilities. As people become more proactive about their health, the market for Influenza A self-test kits is expected to expand, offering a practical solution for early detection and management of the virus. This trend is particularly relevant in the context of global health challenges, where timely diagnosis can significantly influence treatment outcomes and help prevent the spread of the virus.
Colloidal Gold, Fluorescence Probes, Others in the Influenza A Self-test Kits - Global Market:
Colloidal gold, fluorescence probes, and other technologies are integral components of Influenza A self-test kits, each offering unique advantages in the detection process. Colloidal gold is a popular choice due to its simplicity and cost-effectiveness. It involves the use of gold nanoparticles that are conjugated with antibodies specific to the Influenza A virus. When a sample containing the virus is applied to the test strip, the gold nanoparticles bind to the viral antigens, producing a visible color change that indicates a positive result. This method is widely used because it provides rapid results and does not require complex equipment or specialized training. On the other hand, fluorescence probes offer a more sensitive and specific detection method. These probes are designed to emit fluorescence when they bind to the target viral RNA or proteins. The intensity of the fluorescence signal correlates with the amount of virus present in the sample, allowing for quantitative analysis. Although this method requires more sophisticated equipment, it offers higher accuracy and is less prone to false positives or negatives. Other technologies used in self-test kits include lateral flow assays and nucleic acid amplification tests (NAATs). Lateral flow assays are similar to colloidal gold tests but can incorporate various detection labels, such as latex beads or enzymes, to enhance sensitivity. NAATs, such as polymerase chain reaction (PCR), are highly sensitive and specific, capable of detecting even low levels of viral RNA. However, they are more complex and typically require laboratory equipment, making them less suitable for home use. Despite these differences, all these technologies aim to provide reliable and timely results, empowering individuals to make informed decisions about their health. The choice of technology in a self-test kit often depends on factors such as cost, ease of use, and the required level of sensitivity and specificity. As the global market for Influenza A self-test kits continues to grow, manufacturers are likely to explore new technologies and improve existing ones to meet the diverse needs of consumers. This ongoing innovation is crucial for enhancing the accuracy, accessibility, and affordability of self-testing solutions, ultimately contributing to better public health outcomes.
Hospital, Third-Party Testing Agency, Household in the Influenza A Self-test Kits - Global Market:
Influenza A self-test kits are utilized in various settings, including hospitals, third-party testing agencies, and households, each with distinct applications and benefits. In hospitals, these kits serve as a valuable tool for rapid screening of patients presenting with flu-like symptoms. By providing quick results, healthcare professionals can make timely decisions regarding patient management, isolation, and treatment, thereby reducing the risk of nosocomial infections and improving patient outcomes. The use of self-test kits in hospitals also helps alleviate the burden on laboratory services, allowing them to focus on more complex diagnostic tasks. Third-party testing agencies, such as clinics and diagnostic centers, also benefit from the convenience and efficiency of Influenza A self-test kits. These agencies often cater to individuals seeking quick and reliable testing without the need for a hospital visit. By offering self-test kits, they can expand their service offerings and attract a broader clientele, including those who prefer a more private and less time-consuming testing experience. Additionally, third-party testing agencies can use these kits to conduct mass screening programs in community settings, such as schools or workplaces, to identify and manage outbreaks promptly. In households, Influenza A self-test kits empower individuals to take control of their health by enabling them to test for the virus at the first sign of symptoms. This early detection is crucial for initiating timely treatment and preventing the spread of the virus to family members and the wider community. The convenience of home testing also reduces the need for unnecessary doctor visits, saving time and resources for both individuals and healthcare systems. Moreover, self-test kits can provide peace of mind for individuals who may be concerned about potential exposure to the virus, allowing them to confirm their health status quickly and easily. As the demand for accessible and efficient testing solutions continues to rise, the use of Influenza A self-test kits in these various settings is expected to grow, contributing to better health outcomes and more effective management of influenza outbreaks.
Influenza A Self-test Kits - Global Market Outlook:
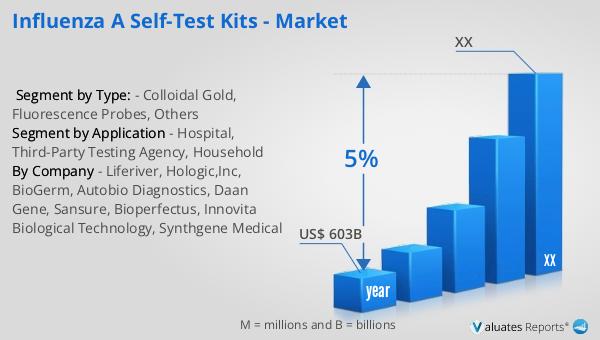
Based on our analysis, the global market for medical devices, which includes Influenza A self-test kits, is projected to reach approximately $603 billion in 2023. This substantial market size reflects the increasing demand for innovative healthcare solutions that cater to the evolving needs of consumers and healthcare providers. Over the next six years, the market is anticipated to grow at a compound annual growth rate (CAGR) of 5%. This growth trajectory underscores the expanding role of medical devices in enhancing healthcare delivery and improving patient outcomes. The rising prevalence of chronic diseases, an aging population, and the growing emphasis on preventive healthcare are key factors driving this market expansion. Additionally, technological advancements and the increasing adoption of digital health solutions are expected to further propel market growth. As healthcare systems worldwide strive to become more efficient and patient-centric, the demand for medical devices, including self-test kits, is likely to continue its upward trend. This positive market outlook highlights the significant opportunities for manufacturers and stakeholders in the medical device industry to innovate and deliver solutions that address the diverse needs of patients and healthcare providers.
| Report Metric | Details |
| Report Name | Influenza A Self-test Kits - Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Liferiver, Hologic,Inc, BioGerm, Autobio Diagnostics, Daan Gene, Sansure, Bioperfectus, Innovita Biological Technology, Synthgene Medical |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
