What is Global Transfusion Safety Diagnostics Market?
The Global Transfusion Safety Diagnostics Market is a crucial segment within the healthcare industry, focusing on ensuring the safety and efficacy of blood transfusions. This market encompasses a range of products and technologies designed to test and screen blood and blood components before transfusion. The primary goal is to prevent the transmission of infectious diseases and ensure compatibility between donor and recipient blood types. With the increasing demand for blood transfusions due to rising surgical procedures, trauma cases, and chronic diseases, the importance of transfusion safety diagnostics has grown significantly. These diagnostics help in detecting pathogens such as HIV, hepatitis B and C, syphilis, and other transfusion-transmissible infections. The market is driven by technological advancements, increasing awareness about blood safety, and stringent regulatory requirements. As healthcare systems worldwide strive to improve patient outcomes, the role of transfusion safety diagnostics becomes even more critical. The market is characterized by continuous innovation, with companies investing in research and development to introduce more accurate and faster diagnostic solutions. Overall, the Global Transfusion Safety Diagnostics Market plays a vital role in enhancing the safety and reliability of blood transfusions, ultimately contributing to better healthcare delivery.
Instruments, Kits & Reagents in the Global Transfusion Safety Diagnostics Market:
In the Global Transfusion Safety Diagnostics Market, instruments, kits, and reagents form the backbone of the testing and screening processes. Instruments used in this market include advanced analyzers and automated systems that facilitate the efficient and accurate testing of blood samples. These instruments are designed to handle high volumes of samples, ensuring quick turnaround times, which is crucial in emergency situations. They are equipped with sophisticated software that allows for precise data analysis and reporting, enhancing the reliability of test results. Kits and reagents, on the other hand, are essential components used in the actual testing process. They include a variety of assays and chemicals that react with blood samples to detect the presence of pathogens or to determine blood compatibility. These kits are designed to be user-friendly, allowing laboratory technicians to perform tests with ease and accuracy. The reagents are formulated to provide high sensitivity and specificity, ensuring that even low levels of pathogens can be detected. In recent years, there has been a significant focus on developing multiplex kits that can test for multiple pathogens simultaneously, thereby increasing efficiency and reducing costs. The integration of advanced technologies such as polymerase chain reaction (PCR) and next-generation sequencing (NGS) in these kits has further enhanced their diagnostic capabilities. PCR-based kits are particularly popular due to their ability to amplify small amounts of DNA, making it easier to detect infections at an early stage. NGS, on the other hand, offers comprehensive insights into the genetic makeup of pathogens, aiding in the identification of new or emerging infectious agents. The market for instruments, kits, and reagents is highly competitive, with numerous players striving to innovate and improve their offerings. Companies are focusing on developing products that are not only accurate but also cost-effective, catering to the needs of both developed and developing regions. The demand for these products is also influenced by regulatory standards, as governments and health organizations mandate rigorous testing protocols to ensure blood safety. As a result, manufacturers are required to adhere to strict quality control measures and obtain necessary certifications before their products can be used in clinical settings. The growing emphasis on personalized medicine and precision diagnostics is also shaping the development of instruments, kits, and reagents in the transfusion safety diagnostics market. Personalized medicine involves tailoring medical treatment to the individual characteristics of each patient, and accurate diagnostics are a critical component of this approach. By providing detailed information about a patient's blood type and potential infections, these diagnostic tools enable healthcare providers to make informed decisions about transfusion strategies. In conclusion, instruments, kits, and reagents are integral to the Global Transfusion Safety Diagnostics Market, driving advancements in blood testing and screening. Their continued evolution is essential to meet the increasing demand for safe and effective blood transfusions worldwide.
Hospitals, Blood Banks, Diagnostic Laboratories, Plasma Fractionation Companies, Others in the Global Transfusion Safety Diagnostics Market:
The Global Transfusion Safety Diagnostics Market finds extensive usage across various healthcare settings, including hospitals, blood banks, diagnostic laboratories, plasma fractionation companies, and other related facilities. In hospitals, transfusion safety diagnostics are crucial for ensuring that patients receive compatible and safe blood transfusions. Hospitals often deal with a high volume of transfusions, especially in departments such as surgery, emergency care, and oncology. The use of advanced diagnostic tools helps in quickly identifying any potential risks associated with transfusions, thereby minimizing the chances of adverse reactions and improving patient outcomes. Blood banks are another critical area where transfusion safety diagnostics play a vital role. These facilities are responsible for collecting, testing, and storing blood and blood components for future use. The implementation of stringent testing protocols using reliable diagnostic tools ensures that the blood supply remains safe and free from infectious agents. This is particularly important in maintaining public trust and ensuring the availability of safe blood for transfusions. Diagnostic laboratories also rely heavily on transfusion safety diagnostics to conduct comprehensive testing of blood samples. These laboratories often work in collaboration with hospitals and blood banks to provide accurate and timely test results. The use of sophisticated instruments and reagents allows for the detection of a wide range of pathogens, ensuring that any potential threats are identified and addressed promptly. Plasma fractionation companies, which specialize in separating plasma from whole blood and extracting valuable proteins, also benefit from transfusion safety diagnostics. These companies require precise testing to ensure that the plasma products they produce are safe for therapeutic use. By employing advanced diagnostic techniques, they can detect any contaminants or impurities, thereby maintaining the quality and safety of their products. Other areas where transfusion safety diagnostics are utilized include research institutions and public health organizations. These entities often conduct studies and surveillance programs to monitor the prevalence of transfusion-transmissible infections and develop strategies to mitigate their impact. The data obtained from diagnostic tests is invaluable in shaping public health policies and improving blood safety standards. Overall, the usage of transfusion safety diagnostics across these various settings underscores their importance in ensuring the safety and efficacy of blood transfusions. As the demand for blood and blood products continues to rise, the role of these diagnostics in safeguarding public health becomes increasingly critical.
Global Transfusion Safety Diagnostics Market Outlook:
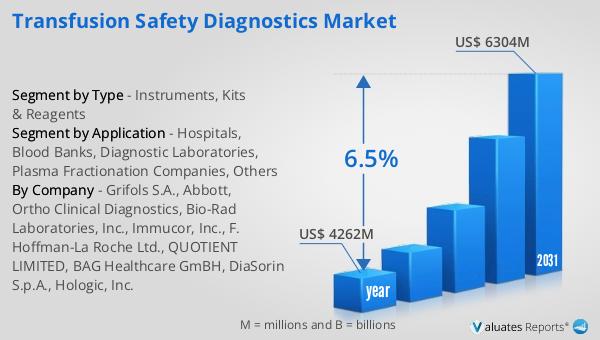
The global market for Transfusion Safety Diagnostics was valued at approximately $4,262 million in 2024, and it is anticipated to grow significantly, reaching an estimated size of $6,304 million by 2031. This growth trajectory represents a compound annual growth rate (CAGR) of 6.5% over the forecast period. The increasing demand for safe and reliable blood transfusions, coupled with advancements in diagnostic technologies, is driving this market expansion. As healthcare systems worldwide continue to prioritize patient safety and improve transfusion practices, the need for accurate and efficient diagnostic tools becomes more pronounced. The market's growth is also supported by the rising prevalence of chronic diseases and the increasing number of surgical procedures, both of which contribute to the demand for blood transfusions. Additionally, the implementation of stringent regulatory standards and guidelines for blood safety further propels the adoption of transfusion safety diagnostics. Companies operating in this market are focusing on innovation and product development to meet the evolving needs of healthcare providers and patients. By investing in research and development, they aim to introduce more advanced and cost-effective diagnostic solutions that enhance the safety and efficacy of blood transfusions. Overall, the Global Transfusion Safety Diagnostics Market is poised for substantial growth, driven by the increasing emphasis on blood safety and the continuous advancements in diagnostic technologies.
| Report Metric | Details |
| Report Name | Transfusion Safety Diagnostics Market |
| Accounted market size in year | US$ 4262 million |
| Forecasted market size in 2031 | US$ 6304 million |
| CAGR | 6.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Grifols S.A., Abbott, Ortho Clinical Diagnostics, Bio-Rad Laboratories, Inc., Immucor, Inc., F. Hoffman-La Roche Ltd., QUOTIENT LIMITED, BAG Healthcare GmBH, DiaSorin S.p.A., Hologic, Inc. |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
