What is PD-L1 and PIK3CA Testing Product - Global Market?
PD-L1 and PIK3CA testing products are crucial components in the field of precision medicine, particularly in oncology. These tests are designed to identify specific biomarkers that can guide treatment decisions for cancer patients. PD-L1, or Programmed Death-Ligand 1, is a protein that plays a significant role in suppressing the immune system, particularly during cancer progression. By testing for PD-L1 expression, healthcare providers can determine whether a patient is likely to benefit from immunotherapy drugs, which are designed to block the interaction between PD-L1 and its receptor, PD-1, thereby enhancing the immune system's ability to attack cancer cells. On the other hand, PIK3CA is a gene that, when mutated, can lead to uncontrolled cell growth and cancer. Testing for PIK3CA mutations helps in identifying patients who may benefit from targeted therapies that specifically inhibit the PI3K/AKT/mTOR pathway, a critical signaling pathway involved in cell growth and survival. The global market for PD-L1 and PIK3CA testing products is expanding rapidly, driven by the increasing prevalence of cancer and the growing adoption of personalized medicine approaches. These tests are becoming integral in tailoring cancer treatment plans to individual patients, thereby improving outcomes and reducing unnecessary side effects.
PD-L1, PIK3CA in the PD-L1 and PIK3CA Testing Product - Global Market:
The PD-L1 and PIK3CA testing product market is a dynamic and rapidly evolving sector within the broader field of molecular diagnostics. PD-L1 testing is primarily used in the context of immuno-oncology, where it serves as a predictive biomarker for the efficacy of immune checkpoint inhibitors. These inhibitors, such as pembrolizumab and nivolumab, have revolutionized cancer treatment by enabling the immune system to recognize and destroy cancer cells more effectively. PD-L1 testing helps identify patients who are most likely to respond to these therapies, thus optimizing treatment strategies and improving patient outcomes. The test is typically performed using immunohistochemistry (IHC) techniques on tumor tissue samples, providing valuable insights into the tumor microenvironment and its interaction with the immune system. Meanwhile, PIK3CA testing focuses on detecting mutations in the PIK3CA gene, which are commonly found in various cancers, including breast, colorectal, and endometrial cancers. These mutations activate the PI3K/AKT/mTOR signaling pathway, promoting tumor growth and survival. By identifying PIK3CA mutations, clinicians can select targeted therapies that inhibit this pathway, such as alpelisib, which has shown efficacy in treating PIK3CA-mutant cancers. The global market for PD-L1 and PIK3CA testing products is driven by several factors, including the rising incidence of cancer, advancements in molecular diagnostic technologies, and the increasing demand for personalized medicine. As more targeted therapies and immunotherapies receive regulatory approval, the need for companion diagnostic tests like PD-L1 and PIK3CA is expected to grow, further fueling market expansion. Additionally, ongoing research and development efforts are focused on improving the accuracy and reliability of these tests, as well as exploring new applications in other cancer types and therapeutic areas. The integration of next-generation sequencing (NGS) technologies and liquid biopsy approaches is also anticipated to enhance the capabilities of PD-L1 and PIK3CA testing, enabling more comprehensive and non-invasive assessments of tumor biology. Overall, the PD-L1 and PIK3CA testing product market represents a critical component of the precision medicine landscape, offering significant potential to improve cancer diagnosis, treatment, and patient outcomes.
Hospital, Diagnostic Center, Others in the PD-L1 and PIK3CA Testing Product - Global Market:
In the context of healthcare settings, PD-L1 and PIK3CA testing products are utilized across various facilities, including hospitals, diagnostic centers, and other specialized institutions. In hospitals, these tests are integral to the oncology departments, where they aid in the diagnosis and treatment planning for cancer patients. Oncologists rely on PD-L1 testing to determine the suitability of immunotherapy for their patients, as it helps identify those who are likely to benefit from immune checkpoint inhibitors. This personalized approach to cancer treatment not only enhances the efficacy of therapy but also minimizes the risk of adverse effects associated with inappropriate treatment choices. Similarly, PIK3CA testing is employed to guide the use of targeted therapies, ensuring that patients with PIK3CA mutations receive the most appropriate and effective treatment options. Diagnostic centers play a crucial role in the widespread adoption of PD-L1 and PIK3CA testing, as they provide the necessary infrastructure and expertise to perform these complex molecular assays. These centers often collaborate with hospitals and clinics to offer comprehensive testing services, enabling timely and accurate diagnosis of cancer patients. By leveraging advanced technologies such as next-generation sequencing and digital pathology, diagnostic centers can deliver high-quality results that inform clinical decision-making and improve patient outcomes. In addition to hospitals and diagnostic centers, other healthcare facilities, such as research institutions and specialized cancer clinics, also utilize PD-L1 and PIK3CA testing products. These institutions often engage in clinical trials and research studies aimed at exploring new applications and improving the performance of these tests. By participating in such initiatives, they contribute to the advancement of precision medicine and the development of novel therapeutic strategies for cancer patients. Overall, the use of PD-L1 and PIK3CA testing products across various healthcare settings underscores their importance in the modern oncology landscape, where personalized medicine is becoming the standard of care.
PD-L1 and PIK3CA Testing Product - Global Market Outlook:
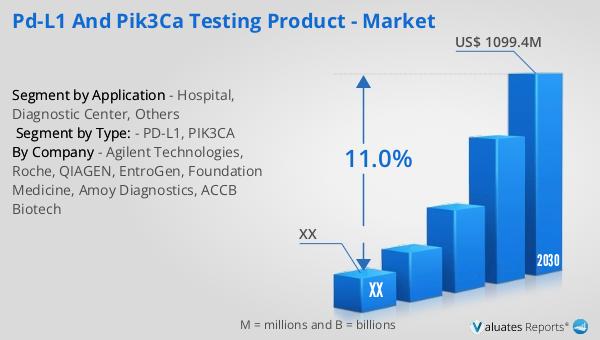
The market outlook for PD-L1 and PIK3CA testing products indicates a robust growth trajectory over the coming years. In 2023, the market was valued at approximately US$ 521.8 million, and it is projected to reach a revised size of US$ 1099.4 million by 2030, reflecting a compound annual growth rate (CAGR) of 11.0% during the forecast period from 2024 to 2030. This significant growth is driven by the increasing adoption of precision medicine approaches in oncology, as well as the rising prevalence of cancer worldwide. North America currently holds the largest share of the market, accounting for nearly 57% of the total consumption. This dominance can be attributed to several factors, including the presence of a well-established healthcare infrastructure, high levels of awareness and adoption of advanced diagnostic technologies, and substantial investments in research and development. The region's strong focus on personalized medicine and the availability of a wide range of targeted therapies and immunotherapies further contribute to the high demand for PD-L1 and PIK3CA testing products. As the market continues to expand, it is expected that other regions, such as Europe and Asia-Pacific, will also experience significant growth, driven by increasing healthcare expenditures, improving access to advanced diagnostic tools, and growing awareness of the benefits of personalized cancer treatment. Overall, the PD-L1 and PIK3CA testing product market is poised for substantial growth, offering numerous opportunities for stakeholders across the healthcare ecosystem to enhance cancer diagnosis and treatment.
| Report Metric | Details |
| Report Name | PD-L1 and PIK3CA Testing Product - Market |
| Forecasted market size in 2030 | US$ 1099.4 million |
| CAGR | 11.0% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Agilent Technologies, Roche, QIAGEN, EntroGen, Foundation Medicine, Amoy Diagnostics, ACCB Biotech |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
