What is Global Cytomegalovirus (CMV) Diagnostic Market?
The Global Cytomegalovirus (CMV) Diagnostic Market is a specialized segment within the broader healthcare diagnostics industry, focusing on the detection and monitoring of cytomegalovirus infections. CMV is a common virus that can cause significant health issues, particularly in individuals with weakened immune systems, such as newborns, organ transplant recipients, and people with HIV/AIDS. The market for CMV diagnostics encompasses a range of tests and technologies designed to identify the presence of the virus in the body, enabling timely and effective treatment. These diagnostics are crucial for preventing the severe complications associated with CMV infections, such as hearing loss, vision impairment, and developmental disabilities in infants. The market is driven by the increasing prevalence of CMV infections, advancements in diagnostic technologies, and growing awareness about the importance of early detection. As healthcare systems worldwide strive to improve patient outcomes and reduce the burden of infectious diseases, the demand for reliable and efficient CMV diagnostic solutions continues to rise. This market is characterized by a diverse array of products, including molecular diagnostics, serological tests, and point-of-care testing kits, each offering unique benefits and applications in various healthcare settings.
Blood, Urine in the Global Cytomegalovirus (CMV) Diagnostic Market:
Blood and urine tests are pivotal components of the Global Cytomegalovirus (CMV) Diagnostic Market, each offering distinct advantages and applications in the detection and management of CMV infections. Blood tests, particularly those based on molecular diagnostics, are highly sensitive and specific, allowing for the accurate detection of CMV DNA in the bloodstream. These tests are essential for diagnosing active CMV infections, especially in immunocompromised patients, where early detection can significantly impact treatment outcomes. Polymerase chain reaction (PCR) assays are commonly used in blood-based diagnostics, providing rapid and reliable results that guide clinical decision-making. Additionally, serological tests, which detect CMV-specific antibodies in the blood, are valuable for determining past exposure to the virus and assessing immunity status. These tests are often used in prenatal screening to identify pregnant women at risk of transmitting the virus to their unborn child. On the other hand, urine tests offer a non-invasive alternative for CMV detection, particularly in newborns and pediatric patients. Urine samples are easy to collect and can be used to identify congenital CMV infections, which are a leading cause of birth defects and developmental disabilities. The use of urine-based diagnostics is especially important in neonatal screening programs, where early identification of CMV can lead to timely interventions that mitigate long-term health impacts. Both blood and urine tests play a crucial role in the comprehensive management of CMV infections, providing healthcare professionals with the tools needed to tailor treatment strategies to individual patient needs. As the CMV diagnostic market continues to evolve, innovations in testing methodologies and the integration of advanced technologies are expected to enhance the accuracy, speed, and accessibility of these diagnostic solutions. The growing emphasis on personalized medicine and precision healthcare further underscores the importance of blood and urine tests in the CMV diagnostic landscape, as they enable clinicians to make informed decisions based on a patient's unique viral load and immune response. Moreover, the increasing adoption of point-of-care testing devices is transforming the way CMV diagnostics are delivered, allowing for rapid on-site testing and immediate results that facilitate prompt clinical interventions. This shift towards decentralized testing is particularly beneficial in resource-limited settings, where access to laboratory infrastructure may be limited. Overall, blood and urine tests are integral to the Global Cytomegalovirus (CMV) Diagnostic Market, providing essential insights into the presence and progression of CMV infections and supporting the delivery of effective, patient-centered care.
Hospitals, Diagnostic Centers and Clinics, Others in the Global Cytomegalovirus (CMV) Diagnostic Market:
The usage of Global Cytomegalovirus (CMV) Diagnostic Market solutions spans various healthcare settings, including hospitals, diagnostic centers and clinics, and other specialized facilities, each playing a vital role in the detection and management of CMV infections. In hospitals, CMV diagnostics are integral to the care of patients with compromised immune systems, such as those undergoing organ transplants or receiving chemotherapy. These patients are at heightened risk for CMV-related complications, making timely and accurate diagnosis crucial for effective treatment. Hospitals often utilize advanced molecular diagnostic techniques, such as PCR assays, to monitor viral load and guide antiviral therapy, ensuring optimal patient outcomes. Additionally, serological tests are employed in prenatal care to assess the risk of congenital CMV transmission, enabling healthcare providers to implement preventive measures and closely monitor at-risk pregnancies. Diagnostic centers and clinics also play a significant role in the CMV diagnostic market, offering a range of testing services to support early detection and disease management. These facilities often serve as the first point of contact for patients experiencing symptoms of CMV infection, providing accessible and efficient testing options that facilitate prompt diagnosis and treatment. The availability of point-of-care testing devices in these settings further enhances the speed and convenience of CMV diagnostics, allowing for rapid on-site testing and immediate results. This is particularly beneficial in outpatient settings, where quick turnaround times are essential for effective patient management. Moreover, diagnostic centers and clinics often collaborate with hospitals and other healthcare providers to ensure continuity of care and comprehensive disease management for CMV-infected patients. Beyond hospitals and diagnostic centers, other specialized facilities, such as research laboratories and public health organizations, contribute to the CMV diagnostic market by advancing our understanding of the virus and developing innovative testing solutions. These entities conduct epidemiological studies to track the prevalence and impact of CMV infections, informing public health strategies and guiding resource allocation. Furthermore, research laboratories are at the forefront of developing novel diagnostic technologies, such as next-generation sequencing and digital PCR, which offer enhanced sensitivity and specificity for CMV detection. The integration of these cutting-edge technologies into routine clinical practice holds the potential to revolutionize CMV diagnostics, improving the accuracy and efficiency of testing and ultimately enhancing patient care. Overall, the Global Cytomegalovirus (CMV) Diagnostic Market is characterized by a diverse array of applications across various healthcare settings, each contributing to the early detection and effective management of CMV infections. As the market continues to evolve, ongoing collaboration between healthcare providers, researchers, and industry stakeholders will be essential to drive innovation and improve patient outcomes.
Global Cytomegalovirus (CMV) Diagnostic Market Outlook:
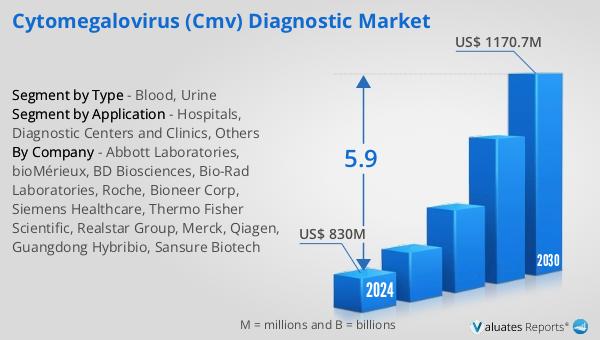
The outlook for the Global Cytomegalovirus (CMV) Diagnostic Market indicates a promising growth trajectory, with projections suggesting an increase from $830 million in 2024 to $1,170.7 million by 2030, reflecting a compound annual growth rate (CAGR) of 5.9% during the forecast period. This growth is indicative of the rising demand for CMV diagnostic solutions, driven by factors such as the increasing prevalence of CMV infections, advancements in diagnostic technologies, and growing awareness about the importance of early detection. In comparison, the global pharmaceutical market, valued at $1,475 billion in 2022, is expected to grow at a CAGR of 5% over the next six years, highlighting the robust expansion of the healthcare sector as a whole. Meanwhile, the chemical drug market, which was estimated at $1,005 billion in 2018, reached $1,094 billion by 2022, underscoring the steady growth of pharmaceutical products. These figures illustrate the dynamic nature of the healthcare industry, with the CMV diagnostic market emerging as a key area of focus due to its critical role in managing infectious diseases. As healthcare systems worldwide continue to prioritize patient outcomes and invest in innovative diagnostic solutions, the CMV diagnostic market is poised for significant growth, offering new opportunities for industry stakeholders and improving the quality of care for patients affected by CMV infections.
| Report Metric | Details |
| Report Name | Cytomegalovirus (CMV) Diagnostic Market |
| Accounted market size in 2024 | US$ 830 in million |
| Forecasted market size in 2030 | US$ 1170.7 million |
| CAGR | 5.9 |
| Base Year | 2024 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Abbott Laboratories, bioMérieux, BD Biosciences, Bio-Rad Laboratories, Roche, Bioneer Corp, Siemens Healthcare, Thermo Fisher Scientific, Realstar Group, Merck, Qiagen, Guangdong Hybribio, Sansure Biotech |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
