What is Global Enterovirus Diagnostic Market?
The Global Enterovirus Diagnostic Market is a specialized segment within the broader healthcare diagnostics industry, focusing on the identification and analysis of enteroviruses. These viruses are a group of RNA viruses that include polioviruses, coxsackieviruses, echoviruses, and other enteroviruses, which can cause a range of illnesses from mild respiratory infections to more severe conditions like meningitis and myocarditis. The market for enterovirus diagnostics is driven by the need for accurate and timely detection of these viruses to prevent outbreaks and manage patient care effectively. With advancements in diagnostic technologies, the market has seen the introduction of more sophisticated and rapid testing methods, which have improved the accuracy and speed of diagnosis. The increasing prevalence of enterovirus infections, coupled with a growing awareness of the importance of early diagnosis, has fueled the demand for enterovirus diagnostic solutions globally. Additionally, the market is influenced by factors such as healthcare infrastructure development, government initiatives for disease control, and the rising incidence of viral infections. As a result, the Global Enterovirus Diagnostic Market is poised for significant growth, reflecting the critical role of diagnostics in managing public health challenges.
Viral Isolation Testing, Serology Testing, Polymerase Chain Reaction (PCR) Assay in the Global Enterovirus Diagnostic Market:
Viral Isolation Testing, Serology Testing, and Polymerase Chain Reaction (PCR) Assay are pivotal components of the Global Enterovirus Diagnostic Market, each offering unique advantages in the detection and analysis of enteroviruses. Viral Isolation Testing is a traditional method that involves culturing the virus in a laboratory setting to confirm its presence. This method, while time-consuming, is highly specific and can provide detailed information about the virus, including its strain and characteristics. It is often used as a confirmatory test following initial screenings. However, the requirement for specialized laboratory facilities and skilled personnel can limit its widespread application, especially in resource-constrained settings. On the other hand, Serology Testing involves the detection of antibodies in the blood, indicating a past or current infection. This method is less invasive and can be performed relatively quickly, making it suitable for large-scale screenings. Serology tests are particularly useful in epidemiological studies to understand the spread of enteroviruses within populations. However, they may not always distinguish between different types of enteroviruses, which can be a limitation in certain clinical scenarios. The Polymerase Chain Reaction (PCR) Assay is a more modern and highly sensitive technique that amplifies viral genetic material, allowing for the detection of even small amounts of the virus in a sample. PCR assays are known for their rapid turnaround times and high accuracy, making them a preferred choice in clinical diagnostics. They can identify specific enterovirus strains, which is crucial for targeted treatment and outbreak management. Despite their advantages, PCR assays require sophisticated equipment and technical expertise, which can be a barrier in some healthcare settings. Each of these diagnostic methods plays a vital role in the comprehensive approach to enterovirus detection, with their combined use enhancing the overall effectiveness of the Global Enterovirus Diagnostic Market. As technology continues to advance, these methods are expected to evolve, offering even greater precision and efficiency in the fight against enterovirus-related diseases.
Hospitals, Laboratories, Others in the Global Enterovirus Diagnostic Market:
The Global Enterovirus Diagnostic Market finds significant application across various healthcare settings, including hospitals, laboratories, and other medical facilities. In hospitals, enterovirus diagnostics are crucial for the timely identification and management of infections, particularly in pediatric and neonatal care units where patients are more vulnerable to severe complications. Rapid and accurate diagnosis in hospital settings enables healthcare providers to implement appropriate treatment plans, reduce the risk of transmission, and improve patient outcomes. Hospitals often rely on a combination of diagnostic methods, such as PCR assays and serology tests, to ensure comprehensive screening and monitoring of enterovirus infections. In laboratories, enterovirus diagnostics play a critical role in research and epidemiological studies. Laboratories are equipped with advanced technologies and skilled personnel to conduct detailed analyses of enterovirus samples, contributing to a better understanding of the virus's behavior, transmission patterns, and genetic variations. This information is invaluable for developing effective public health strategies and vaccines. Laboratories also serve as reference centers for confirmatory testing, supporting hospitals and other healthcare facilities in complex cases. Beyond hospitals and laboratories, the Global Enterovirus Diagnostic Market extends to other areas such as clinics, research institutions, and public health organizations. These entities utilize enterovirus diagnostics for various purposes, including routine screenings, outbreak investigations, and surveillance programs. Public health organizations, in particular, rely on diagnostic data to monitor enterovirus activity, assess the effectiveness of control measures, and allocate resources efficiently. The versatility and adaptability of enterovirus diagnostics across different settings underscore their importance in safeguarding public health and preventing the spread of infections. As the demand for enterovirus diagnostics continues to grow, driven by increasing awareness and technological advancements, these tools will remain integral to healthcare systems worldwide.
Global Enterovirus Diagnostic Market Outlook:
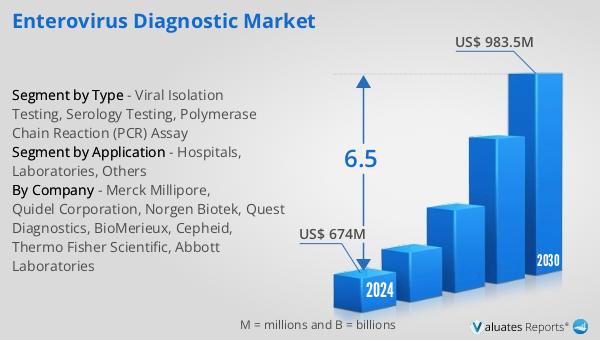
The outlook for the Global Enterovirus Diagnostic Market indicates a promising growth trajectory, with the market expected to expand from $674 million in 2024 to $983.5 million by 2030, reflecting a Compound Annual Growth Rate (CAGR) of 6.5% during the forecast period. This growth is indicative of the increasing demand for effective diagnostic solutions to manage enterovirus infections, which pose significant public health challenges. The broader pharmaceutical market, valued at $1,475 billion in 2022, is also experiencing growth, albeit at a slightly lower CAGR of 5% over the next six years. This expansion highlights the overall positive trend in the healthcare sector, driven by advancements in medical technology and an increasing focus on disease prevention and management. In comparison, the chemical drug market has shown a steady increase from $1,005 billion in 2018 to $1,094 billion in 2022, underscoring the ongoing demand for pharmaceutical products. The growth of the Global Enterovirus Diagnostic Market is a testament to the critical role of diagnostics in enhancing healthcare outcomes and addressing the evolving needs of patients and healthcare providers. As the market continues to evolve, it is expected to contribute significantly to the broader healthcare landscape, supporting efforts to improve public health and combat infectious diseases.
| Report Metric | Details |
| Report Name | Enterovirus Diagnostic Market |
| Accounted market size in 2024 | US$ 674 in million |
| Forecasted market size in 2030 | US$ 983.5 million |
| CAGR | 6.5 |
| Base Year | 2024 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Merck Millipore, Quidel Corporation, Norgen Biotek, Quest Diagnostics, BioMerieux, Cepheid, Thermo Fisher Scientific, Abbott Laboratories |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
