What is Global Cytomegalovirus (CMV) Vaccine Market?
The Global Cytomegalovirus (CMV) Vaccine Market is a specialized segment within the broader pharmaceutical industry, focusing on the development and distribution of vaccines aimed at preventing infections caused by the cytomegalovirus. CMV is a common virus that can infect people of all ages, but it is particularly concerning for pregnant women, newborns, and individuals with weakened immune systems. The market for CMV vaccines is driven by the increasing awareness of the virus's potential health impacts and the ongoing research and development efforts to create effective vaccines. As of now, there is no approved CMV vaccine available, but several candidates are in various stages of clinical trials. The market is characterized by significant investments from pharmaceutical companies and research institutions, aiming to address the unmet medical need for a CMV vaccine. The growth of this market is also influenced by advancements in vaccine technology and the increasing prevalence of CMV infections globally. As the demand for effective preventive measures against CMV rises, the market is expected to expand, offering new opportunities for stakeholders involved in vaccine development and distribution.
Attenuated Vaccines, Subunit Vaccines in the Global Cytomegalovirus (CMV) Vaccine Market:
Attenuated vaccines and subunit vaccines are two prominent types of vaccines being explored in the Global Cytomegalovirus (CMV) Vaccine Market. Attenuated vaccines are created by reducing the virulence of a pathogen, but still keeping it viable, or "live." These vaccines aim to elicit a strong and lasting immune response by mimicking a natural infection. In the context of CMV, attenuated vaccines are designed to introduce a weakened form of the virus to the immune system, prompting it to recognize and combat the virus without causing the disease itself. This approach has been successful in other vaccines, such as those for measles and mumps, and holds promise for CMV. However, the development of attenuated CMV vaccines poses challenges, including ensuring the safety and stability of the vaccine, especially for immunocompromised individuals who may be at risk from even a weakened virus. On the other hand, subunit vaccines represent a different strategy in the CMV vaccine market. These vaccines use specific pieces of the virus, such as proteins or glycoproteins, to stimulate an immune response. By focusing on particular components of the virus, subunit vaccines can target the immune system more precisely, potentially reducing the risk of side effects associated with live vaccines. In the case of CMV, subunit vaccines often focus on the glycoprotein B (gB) and the pentameric complex, which are crucial for the virus's ability to infect cells. These components are used to train the immune system to recognize and neutralize the virus. Subunit vaccines are generally considered safer for individuals with weakened immune systems, as they do not contain live virus particles. However, they may require adjuvants to enhance the immune response and often necessitate multiple doses to achieve optimal protection. The development of both attenuated and subunit vaccines for CMV is a complex process that involves extensive research and clinical testing. Researchers must balance the need for a strong immune response with the safety and tolerability of the vaccine. The choice between attenuated and subunit vaccines depends on various factors, including the target population, the desired immune response, and the potential risks associated with each type of vaccine. As the CMV vaccine market continues to evolve, both approaches are being explored to provide effective and safe options for preventing CMV infections. The ongoing advancements in vaccine technology and a deeper understanding of the virus's biology are expected to drive innovation in this field, ultimately leading to the development of a successful CMV vaccine.
Laboratories, Hospitals, Diagnostic Centers and Clinics, Others in the Global Cytomegalovirus (CMV) Vaccine Market:
The usage of the Global Cytomegalovirus (CMV) Vaccine Market spans various healthcare settings, including laboratories, hospitals, diagnostic centers, clinics, and other medical facilities. In laboratories, the focus is primarily on research and development activities related to CMV vaccines. Scientists and researchers work tirelessly to understand the virus's structure, behavior, and interaction with the human immune system. This research is crucial for identifying potential vaccine candidates and testing their efficacy and safety in preclinical and clinical trials. Laboratories also play a vital role in developing diagnostic tools that can accurately detect CMV infections, which is essential for monitoring the effectiveness of vaccines once they are available. Hospitals are another critical area where the CMV vaccine market is expected to have a significant impact. Hospitals often deal with patients who are at high risk of CMV infections, such as newborns, pregnant women, and individuals with compromised immune systems. The availability of a CMV vaccine would be a valuable tool for healthcare providers in these settings, helping to prevent infections and reduce the associated health complications. Hospitals may also be involved in clinical trials for CMV vaccines, providing a controlled environment for testing vaccine candidates on human subjects. This involvement is crucial for gathering data on the vaccine's safety and efficacy, which is necessary for regulatory approval and widespread use. Diagnostic centers and clinics also play a vital role in the CMV vaccine market. These facilities are often the first point of contact for individuals seeking information and testing for CMV infections. With the introduction of a CMV vaccine, diagnostic centers and clinics would be instrumental in administering the vaccine to the public. They would also be responsible for educating patients about the benefits and potential side effects of the vaccine, ensuring that individuals make informed decisions about their health. Additionally, these centers would continue to offer diagnostic services to monitor the prevalence of CMV infections and assess the vaccine's impact on public health. Other healthcare settings, such as community health centers and specialized medical facilities, also contribute to the CMV vaccine market. These facilities often serve populations that may not have easy access to larger hospitals or clinics, making them essential for ensuring widespread vaccine coverage. Community health centers, in particular, can play a crucial role in reaching underserved populations and providing education and vaccination services. As the CMV vaccine market continues to develop, these various healthcare settings will be integral to the successful distribution and administration of the vaccine, ultimately helping to reduce the burden of CMV infections worldwide.
Global Cytomegalovirus (CMV) Vaccine Market Outlook:
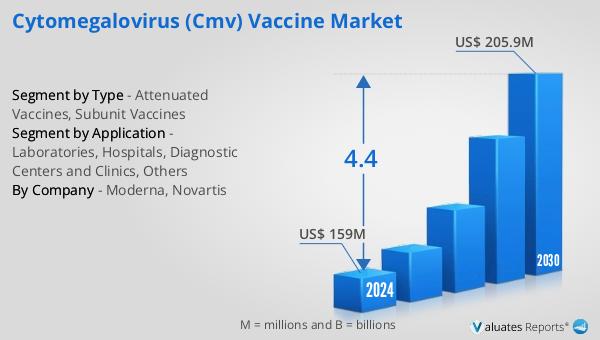
The outlook for the Global Cytomegalovirus (CMV) Vaccine Market indicates a promising growth trajectory. It is anticipated that the market will expand from $159 million in 2024 to $205.9 million by 2030, reflecting a Compound Annual Growth Rate (CAGR) of 4.4% over the forecast period. This growth is indicative of the increasing recognition of the need for a CMV vaccine and the ongoing efforts to develop effective solutions. In the broader context, the global pharmaceutical market was valued at $1,475 billion in 2022 and is projected to grow at a CAGR of 5% over the next six years. This growth underscores the dynamic nature of the pharmaceutical industry and the continuous demand for innovative healthcare solutions. Comparatively, the chemical drug market has shown a steady increase, rising from $1,005 billion in 2018 to $1,094 billion in 2022. These figures highlight the robust expansion of the pharmaceutical sector, driven by advancements in drug development and an increasing focus on addressing unmet medical needs. The CMV vaccine market, as a part of this larger industry, is poised to benefit from these trends, with significant opportunities for growth and innovation.
| Report Metric | Details |
| Report Name | Cytomegalovirus (CMV) Vaccine Market |
| Accounted market size in 2024 | US$ 159 million |
| Forecasted market size in 2030 | US$ 205.9 million |
| CAGR | 4.4 |
| Base Year | 2024 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Segment by Region |
|
| By Company | Moderna, Novartis |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
